MEN'S HEALTH I
CANCER
Advances in surgical treatment for prostate carcinoma
A new group of focused treatments aim to surgically treat disease without removal of the gland
February 4, 2015
-
Surgery plays a key role in the management of prostate cancer, particularly in organ-confined disease, but also in some locally advanced disease and in post-radiotherapy relapse.
The standard approach to surgical management of prostate cancer is radical retropubic prostatectomy (RRP), which involves removal of the entire prostate gland and seminal vesicles, and anastomosis of the urethra to the bladder neck. The basic principles of this surgery have remained largely unchanged for many years, however, there have been certain refinements undertaken to make the surgery less morbid and less invasive. We have also seen the emergence of a new group of ‘focused treatments’ that aim to surgically treat disease without removal of the gland.
Radical prostatectomy
In prostate cancer surgery, the priorities for the surgeon are removal of all malignant tissue, preservation of urinary continence and maintenance of erectile function (where possible), in that order. As such, these endpoints are generally used for comparison of novel surgical techniques.
Minimally invasive surgery for prostate cancer has emerged as a viable alternative to the traditional open operation. This can be performed either by standard laparoscopic means or by a robot-assisted technique. The latter is fast becoming the surgical option of choice in the US. In Ireland, there are currently two da Vinci robots in urological operation and several surgeons offering robotic prostatectomy, while laparoscopic prostatectomy is performed by a number of surgeons also.
Despite being relatively new advances, both laparoscopic and robotic prostatectomy have been shown to result in equivalent oncological outcomes as open surgery. A systematic review by Nevara et al1 showed no significant difference in rates of positive surgical margins or progression-free survival between the three techniques. However, they do note that follow-up beyond five years from robot-assisted surgery was limited to a small number of papers.
Recently, five-year biochemical recurrence rates of 14% and mortality rates of 1% have been reported with robot-assisted surgery,2 while 10-year progression-free survival of 78.1-97.2% (depending on stage) has been shown with the laparoscopic technique.3
In terms of morbidity, lower rates of blood loss and transfusion are reported with robotic surgery compared to RRP,4 as well as a significantly lower incidence of urinary incontinence compared to laparoscopic surgery and RRP.5 Interestingly, while robotic surgery was associated with lower rates of impotence than RRP, no superiority was demonstrated over laparoscopic surgery.6
While there is general agreement that robotic prostatectomy is a valuable surgical technique, the Pasadena Consensus Panel concluded that studies with longer follow-up are required to fully define its role and efficacy.7
Focused treatments
With the increase in opportunistic screening with prostate-specific antigen (PSA) in recent years, we are now diagnosing more and more early-stage, low-grade prostate cancers. As such, there is a drive for the development of low-morbidity definitive treatment strategies. Much research in recent times has concentrated on focused surgical treatments for prostate cancer, in which the pathologic areas of the gland are treated with a minimum of injury to the normal prostate tissue.
Two candidate therapies have emerged to date, namely cryotherapy and high intensity focused ultrasound (HIFU), while vascular-targeted photodynamic therapy remains in the experimental stages. Given that it is targeted at the areas of malignancy specifically, it is imperative that accurate localisation of disease is achieved prior to treatment. This mandates either transperineal template biopsies (which can achieve a sensitivity of up to 90%8) or, if unavailable, new multiparametric MRI and transrectal ultrasound (TRUS) biopsy.9
One of the major difficulties with research in this field is the lack of a consistent definition for biochemical recurrence, with different groups utilising different thresholds. Since not all prostate tissue is destroyed, we would expect there to be a non-zero PSA level following treatment, but how to interpret this level remains unclear.
Cryosurgery
Cryosurgery as a discipline has been practised for many years and involves the sequential freezing and thawing of the prostate gland to cause tissue damage and cell death. The patient is placed in the lateral position, under either general or regional anaesthesia, and a number of cryoprobes (up to 30) are placed transperineally into the prostate gland under TRUS guidance. Argon gas is passed through these probes resulting in rapid cooling of the prostate tissues. A urethral warmer is utilised and all probes must be placed at least 8mm from the urethra to prevent tissue sloughing and urethral injury.
Thermosensors are placed at the external sphincter, at the apex of the gland and along Denonvilliers’ fascia to ensure that the tissues reach a temperature of -40ºC, and are maintained at this level for three minutes. Following this, the argon gas is exchanged for helium resulting in thawing of the probes.
Two freeze-thaw cycles have been shown to result in better oncological outcomes than a single cycle10 as well as tissue destruction at a temperature of -41ºC, rather than the -62ºC needed with a single cycle.11
The European Association of Urology (EAU) identifies this as a potentially useful modality in low-to-intermediate-risk prostate cancer in those with prostate size of less than 40g.9
Cryosurgery has been used both as a primary therapeutic modality in prostate cancer and as a salvage treatment for relapse following radiotherapy.12 In the primary setting, five-year actuarial biochemical recurrence-free survival ranged from 36-61% (low-risk to high-risk disease) and 45-76% depending on whether 0.5 or 1.0ng/ml PSA was used as a threshold.13 The overall rate of positive prostate biopsy after therapy was 18%.13
As expected, the results for salvage cryotherapy for patients with recurrence after radiotherapy are worse, with Williams et al reporting a five-year survival of 39%.14
The major complication associated with cryosurgery is erectile dysfunction, with rates of 93% reported,13 improving to 51% at four years with formal penile rehabilitation.15 Sexual function at three years post-treatment was shown to be superior to patients following external beam radiotherapy. Ahmed et al demonstrated incontinence rates of 5% following primary and 10% following salvage cryosurgery.16 Other complications include tissue sloughing, pelvic pain, urinary retention and, rarely, fistula formation.
High intensity focused ultrasound
High intensity focused ultrasound (HIFU) is the other major modality of focal treatment for prostate cancer under investigation. It is also performed under general anaesthesia by a transperineal approach. High intensity ultrasound waves are focused onto the area of abnormality in the gland, resulting in heating of the tissues and coagulative necrosis. Unfortunately, like cryotherapy, despite having been in use for several years, the quality of evidence for this procedure is lacking, with no randomised controlled trials available.
A recent systematic review of 31 uncontrolled trials reported negative post-treatment biopsy rates of 35-95%, and five-year survival of 61.2-95%.17 Blana et al have shown recurrence rates post-HIFU to be related to the level of the PSA nadir, with those achieving lowest post-op PSA levels having the best outcome. As expected, the prognosis associated with this modality is related to the stage at time of diagnosis, with a biochemical disease-free rate at five years post-treatment varying from 74% for T1c disease to 33% for T3.18
Complications encountered with HIFU include urinary retention, erectile dysfunction, urinary incontinence, UTIs and, rarely, recto-urethral fistula. Cordiero et al report that the incidence of obstructive complications can be reduced by TURP, either at the time of HIFU or postoperatively.17
Vascular-targeted photodynamic therapy
The technique of vascular-targeted photodynamic therapy, while currently still in the experimental stage of development, may in future be added to the range of focal therapies available. This approach aims to destroy cancer cells by the activation of a systemically administered photodynamic agent by local application of LASER light under TRUS guidance,19 allowing targeting of therapy and minimisation of complications.
Despite some encouraging results in the field of focal therapy, both the EAU9 and NICE20 advise that these modalities (in general) remain within the field of clinical trials until longer-term follow-up is available. However, the American Urological Association suggests,21 and the EUA concedes, that cryotherapy may be considered in selected cases, particularly in those unfit for radical surgery or with limited life expectancy.
Radiology
The advent of early detection methods for prostate cancer has led to a requirement for more definitive diagnostic modalities. The traditional imaging sequences are being pressed to offer greater detail and definition so as to have minimally invasive, patient-centred, targeted therapies available to more patients diagnosed with prostate cancer. The latest developments have been focused largely on magnetic resonance imaging (MRI) as well as therapeutic nuclear medicine options.
Multiparametric MRI
Multiparametric MRI is a term that has been adopted to incorporate the multitude of MR-based imaging techniques currently being employed in the detection of prostate cancer, among others. The term is usually employed to describe the use of MR spectroscopy, dynamic contrast-enhanced MR and diffusion-weighted imaging, or variations thereof.
MR spectroscopy
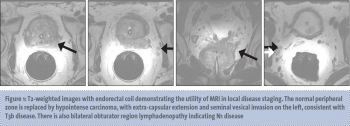
MR spectroscopic imaging (MRSI) generates information about the relative concentrations of specific metabolites within tissues and is commonly performed in 3D imaging of the brain.22 When used for prostate imaging, an endorectal coil is recommended for 3 tesla (3T) magnets and required for 1.5T magnets.23 T2-weighted images are generated (see Figure 1) and are overlaid then by spectral tracings generated by MRSI. MRSI analyses a defined area for specific molecules such as hydrogen ions or protons.
 (click to enlarge)
(click to enlarge)

 (click to enlarge)
(click to enlarge)