CANCER
WOMEN’S HEALTH
Cervical screening: HPV and biomarkers – new options
Incorporation of HPV mRNA and other biomarkers into cervical screening programs has the potential to stratify patients based on their real risk of developing a high-grade lesion or cancer.
February 1, 2012
-
Cervical cancer is the third most frequent malignancy in women worldwide with almost half a million cases diagnosed each year.1 In Ireland, the annual incidence in 2007 was 286 cases, resulting in 83 deaths.2
Cervical cancer is initiated by progression through a well-defined pre-malignant phase known as cervical intraepithelial neoplasia (CIN). This begins as a slow progressive disruption of normal epithelium surrounding the transformation zone. It is staged from CIN1-CIN3 based on the degree of neoplasia, if left untreated lesions can develop into cervical cancer.
In Ireland, the National Cervical Screening Programme, CervicalCheck, was introduced in late 2008 and provides free smear tests for detecting pre-invasive neoplasia to women aged 25-60. Since its launch the programme has proved very successful with an average of 1,000 women availing of a free smear test per day during the first two years of operation. The latest statistics available from the programme indicate that in excess of 280,000 women were screened during the second year, the majority of whom (85%) had a negative smear test; of the remaining 15%, low-grade abnormalities accounted for 12.5% and high-grade abnormalities 1.7%. In total, 16,811 women attended a colposcopy clinic for the first time, with 7,546 receiving treatment.2
HPV infection
Human papillomavirus (HPV) is the single most important aetiological agent in the pathogenesis of cervical cancer and pre-cancer. There are 15-20 types associated with cervical cancer, of which HPV 16, 18, 31, and 45 account for 80% of cervical cancers.3 Up to 80% of sexually active women will acquire a HPV infection at some stage in their lifetime.
HPV infection is more common in younger women, reaching a peak of approximately 20% among women between 20 and 24 years of age, with a subsequent decline among women over 30 years of age.4 In Ireland, HPV prevalence is estimated at 19% in women aged 25-60 years and as high as 30% in women under 30 years of age.5
Secondary to HPV infection, cigarette smoking is one of the main risk factors associated with progression of lesions and development of high-grade CIN.6 The high prevalence of smoking among young women will have a profound effect on future incidence of cervical cancer.
Cytology screening based on the Pap smear is the method used for cervical screening, however, the Pap test is a subjective test with many limitations, specifically in relation to diagnosis of low-grade abnormalities LSIL (low-grade squamous intraepithelial lesions ) and ASCUS (atypical squamous cells of undetermined significance).7 Issues including low sensitivity, equivocal results and inability to distinguish progressive disease have provoked the use of HPV tests in screening programs. It is now widely accepted that HPV testing has a role in cervical cancer prevention in both the pre and post HPV vaccination era.
The roles include:
- As a primary screening tool in women over 30 years
- For triage of women with low grade abnormalities; and
- In the follow-up of women treated for cervical intraepithelial neoplasia (CIN).8
CervicalCheck, like other screening programmes internationally, is due to introduce HPV testing into the Irish screening programme. Since autumn 2011, HPV testing has been used in conjunction with cytology in the follow-up of women treated for CIN. HPV DNA testing is currently used in cervical screening in the United States9 and has recently been approved for triage of LSIL/ASCUS in the UK.10
Issues in management
A significant proportion of cervical abnormalities will regress spontaneously and do not require treatment, however, currently there is no test to distinguish between those that will progress to malignancy and those women that will regress. There remains considerable controversy over the appropriate management of low-grade abnormalities with standard management consisting of cytology screening and/or colposcopic referral. The proportion of women presenting with persistent low-grade abnormalities (LSIL/ASCUS) that go on to develop a high-grade lesion is in the region of 28%. There is a clear need to develop an improved screening algorithm to identify which of these low-grade lesions will progress to a high-grade lesion.
HPV DNA testing, although useful, is still likely to result in over-treatment and unnecessary follow-up of women with low-grade abnormalities, given the high prevalence of transient HPV infection among sexually active women.
The Irish Cervical Screening Research Consortium (CERVIVA) has demonstrated that HPV DNA testing, while it seems to be more sensitive than cytology, is lacking in specificity. In a cross-sectional study of women referred to colposcopy with low-grade abnormalities, the sensitivity of HPV DNA testing for detection of high-grade disease was 90% (95% CI 0.8168-0.9569) and specificity 55% (95% CI 0.4849-0.6327).
There is similar evidence from the ALTS Study (US) and the TOMBOLA study (UK), that HPV DNA testing in particular in low-grade cytology is of limited value.11,12 Early evidence suggests that detection of HPV E6/E7 mRNA is more indicative of a clinically significant infection than a HPV DNA testing approach.13,14
Incorporation of HPV mRNA and other biomarkers into cervical screening programs has the potential to stratify patients based on their real risk of developing a high-grade lesion or cancer.
Approaches to HPV testing
There are three basic approaches to HPV testing:
- HPV DNA testing
- HPV genotyping
- HPV mRNA testing.
HPV DNA testing and genotyping methods are all based on detection of the L1 region of the HPV genome and will not distinguish between transient and integrated oncogene expressing HPV infection.
Persistent infection can lead to integration into the host genome, these transforming infections are considerably more likely to develop high-grade CIN and initiate malignant transformation than women who clear the infection.
HPV mRNA tests detect the viral transforming genes HPV E6/E7. The E6 oncogene interacts with cellular factors, primarily p53, resulting in deregulation of the cell cycle and DNA repair mechanism. E7 targets pRB disrupting its association with transcription factor E2F, resulting in transactivation of genes involved in DNA synthesis and progression through S phase including p16INK4a, TOPO2A, MCMs.3 Evidence to date indicates that HPV mRNA detection has the potential to offer a more specific test for identifying women at risk of developing high-grade disease.14-16 The data suggests that HPV mRNA testing has an overall specificity of between 76-86% for detection of CIN2+. However, many of these studies focus on women in a colposcopy setting. There are few large scale studies that concentrate on HPV mRNA testing as a triage for LSIL/ASCUS.
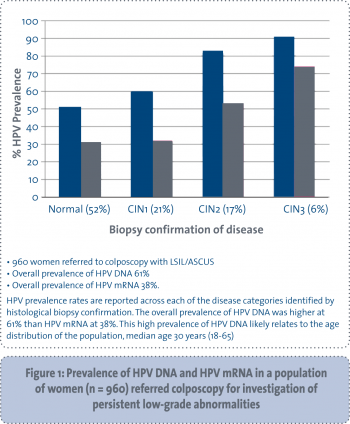
CERVIVA has so far examined the HPV status of 960 women between the ages of 18 and 65 years (median age 30 years), referred to colposcopy for investigation of persistent LSIL/ASCUS. The HPV DNA and mRNA prevalence at first visit to colposcopy is shown in Figure 1. In contrast to HPV DNA, the prevalence of HPV mRNA in this population was 38% compared to 61% for HPV DNA. CERVIVA also established that detection of HPV mRNA appears to be more specific, 79% (95% CI 0.7277-0.8495) than HPV DNA, 55% (95% CI 0.4849-0.6327) for detection of high-grade disease. However, mRNA testing alone is not optimal, since sensitivity (71%) is lower than DNA testing and cytology.
 (click to enlarge)
(click to enlarge)

 (click to enlarge)
(click to enlarge)