CANCER
CERVIVA human papillomavirus primary screening pilot study
Cervical cancer is the fourth most common cancer in women worldwide
August 14, 2017
-
Cervical cancer is the fourth most common cancer in women worldwide.1 In Ireland approximately 300 women are diagnosed with cervical cancer each year, with over 90 deaths. In addition, around 6,500 women require treatment for cervical intraepithelial neoplasia (CIN).2,3
Infection with human papillomavirus (HPV) is the single most important aetiological agent in the pathogenesis of cervical cancer and pre-cancer. It is now increasingly recognised that HPV is also causally implicated in other cancers, including head and neck, vulval, penile and anal cancer.4
There are more than 150 different types of HPV, 40 of which are found to infect the genital tract. A number of these are known as ‘oncogenic HPV types’ (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) based on their potential to cause cervical precancerous abnormalities and cervical cancer.5 Of these oncogenic HPV types, HPV 16 and HPV 18 are associated with approximately 70% of all cervical cancer cases.6
Infection with HPV is necessary, but not sufficient for the development of cervical cancer. Mild cellular changes and mild dysplasia (CIN 1) may occur after an acute HPV infection, but approximately 90% of these will regress without any treatment.7 However, persistent HPV infection may lead to precancerous cellular changes (CIN 2 and CIN 3), a proportion of which will progress, if not treated, to invasive cervical cancer over a period of 10 to 20 years.
Cervical cancer prevention strategies
Optimal cervical cancer prevention requires both effective vaccination and screening. As the vaccines offered in the school-based programmes prevent most but not all high-risk HPV (hrHPV) infections, HPV-immunised women should continue to participate in screening.
Population-based cervical screening programmes operate in many European countries, including Ireland where the CervicalCheck programme has been offering free smear tests to all women aged 25-60 years since September 2008. To date, more than one million women have been screened as part of this programme. In addition, HPV vaccination programmes are established in several countries worldwide7 including Ireland, where a national school-based HPV immunisation programme began in 2010, using the quadrivalent Gardasil HPV vaccine, which protects against HPV types 16, 18, 6 and 11.
Vaccination is offered to girls (approximately 12 years old) with catch-up immunisation (approximately 18 years old) provided from 2011 onwards. For the first four years of the programme, uptake was high [85% among 12-year-olds, and 71% for catch-up cohorts].8 However, this has fallen by 15% in the 2015/2016 cohorts and further again in 2016/2017 cohorts, where uptake is now less than 50%.
This decline in uptake is attributed to unsubstantiated claims regarding the safety of the vaccine. The vaccine is considered safe and well tolerated. In March 2015 the US CDC reported that ‘HPV vaccines are safe and effective vaccine’.9 In November 2015 the European Medicines Agency (EMA) reported on a review of HPV vaccines. This report found no evidence the vaccine was linked to chronic fatigue-like conditions.10
Management of cervical disease in this context is very challenging, as the population becomes stratified into distinct risk groups: vaccinated/screened; vaccinated/unscreened; unvaccinated/screened; and unvaccinated /unscreened.
The changing face of cervical screening
Cervical screening aims to detect women with high-grade precancerous changes that can then be treated, reducing the risk of cervical cancer. Cervical cytology is the traditional technique for screening cervical smears to detect and treat cervical pre-cancer. As with any screening test, it can have false negatives and false positives and its use has to balance sensitivity (the ability of a test to detect disease) and specificity (the likelihood of a positive test identifying underlying disease).
Over the past five years, HPV testing has been introduced into the management of cervical pre-cancer, for example, HPV testing is used to triage women with low-grade cytological abnormalities, and to manage women following treatment for cervical intraepithelial neoplasia (CIN).11
It is likely that the use of HPV testing in screening will assume even more importance as increasing proportions of women who have been vaccinated against HPV enter the screening population.
More recently, there has been a shift towards the use of HPV testing for primary cervical screening as it is significantly more sensitive and has a higher negative predictive value than cytology based primary screening. However, HPV testing for primary screening is a ‘test of risk’ rather than a ‘test of disease’.
In 2015, the HSE National Screening Service requested the Health Information and Quality Authority (HiQA) to carry out a health technology assessment (HTA) on the clinical and cost effectiveness of using HPV testing as the primary screening method instead of cytology. This assessment, which published its findings at the end of May this year, concluded that changing to primary HPV testing would reduce the number of screenings each woman has in her lifetime, while providing better accuracy in detecting precancerous abnormalities and early stage invasive cervical cancer. HiQA has therefore advised that CervicalCheck changes its primary screening method to HPV testing.
This is not without its challenges. While HPV DNA testing has a very high negative predictive value, it has a low specificity or high incidence of false positives.10 Appropriate protocols to stratify HPV-positive women are essential to avoid over-referral and over-treatment.
Discussions on how and when to implement a change in the cervical screening technology will take place between the Department of Health, the HSE and the National Screening Service over the course of the coming months.
Molecular biomarkers as ‘tests of disease’
HPV DNA testing as a primary screening test is more sensitive than cytology for identifying women who have CIN2+, but the specificity is lower.12,13 While high sensitivity is important, many CIN2 and some CIN3 lesions will spontaneously regress, it is therefore possible that tests with lower sensitivity will still identify the lesions which progress to cancer.
Finding a balance between sensitivity and specificity is paramount in the context of primary screening to avoid large numbers of unnecessary testing and follow-up of HPV-positive women. This could be achieved by avoiding screening in younger women (eg. < 30 years), using more specific HPV tests and using appropriate triage algorithms.
The majority of evidence from HPV primary screening RCTs suggests that reflex cytology is a good option for triage of HPV-positive women. However, challenges remain on how to manage women who are HPV positive with a negative cytology result. An alternative approach is to triage with some form of secondary biomarker(s). Several biomarker options exist for this including:
• Detection of HPV E6/E7 mRNA
• Genotyping for HPV16/18
• Co-expression of p16INK4a/Ki-6714,15
• Detection of a panel of methylation biomarkers (eg. CADM1, MAL, miR124).16
Alternative biomarkers have potential to offer more specific triage of HPV positive women. It is known that HPV subtypes 16 and 18 are associated with more than 70% of cervical cancer and consequently induce a higher risk of malignancy. Furthermore, over expression of viral oncogenes E6 and E7 are necessary for malignant transformation. Detection of these oncogenes by the presence of their mRNA transcripts allows better distinction between transient HPV infections and those persistent or active infections that are likely to progress to a cancerous lesion.
The presence of active HPV infections can also be identified through over expression of p16, which plays a major role in cell cycle regulation and Ki-67, a proliferation marker. Over expression of p16 signals E7 mediated deregulation of the cell cycle and thus acts a surrogate marker for active HPV infection. More recently methylation of particular genes has been found to be linked to high-grade pre-cancer and cervical cancer.
CERVIVA, through its HRB-funded CARG (CARG29/2012) programme, is evaluating this range of triage options to stratify women with a HPV positive primary screening smear result.
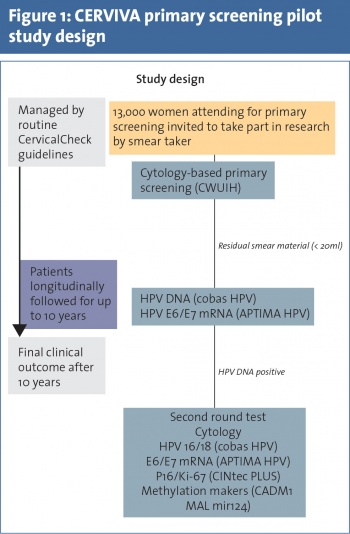
CERVIVA HPV Primary Screening Pilot Study
Molecular triage strategies for HPV-positive women
CERVIVA, in partnership with CervicalCheck, are currently undertaking a HPV primary screening study which is evaluating and comparing different strategies for the triage of women with a HPV-positive primary screening test. This study, funded by the Health Research Board, is an observational cohort study and is aiming to recruit 13,000 women attending primary care for their routine CervicalCheck smear test (see Figure 1). Women attending for their routine CervicalCheck smear tests are invited to participate in the study and asked to give written informed consent.
 (click to enlarge)
(click to enlarge)
