CANCER
Diagnostic radiation dose and oncology patients
With advances in cancer treatment, often leading to cure, the potential hazard of ionising radiation associated with diagnostic imaging in patients with malignant disease is an increasingly important issue
February 1, 2013
-
Medical imaging plays a central and ever-increasing role in the care of the cancer patient. Imaging is used, not only in diagnosis, staging and follow-up of disease and monitoring of treatment response and complications, but also in the interventional setting for the insertion of central venous access devices, tissue biopsy, cavity drainage and with evolving therapeutic roles in the fields of chemoembolisation and radioembolisation. Many of these are relatively low radiation dose procedures and performed on a once-off or periodic basis. Computed tomography (CT) and positron emission tomography (PET CT) have become indispensable tools in oncological imaging, both resulting in exposure of the patient to significant doses of ionising radiation, with CT being the single biggest contributor to dose in diagnostic imaging in current practice.1
Radiation exposure in oncology patients
Detrimental effects of ionising radiation comprise deterministic and stochastic effects. Deterministic effects are predictable, usually limited by thresholds, and become worse with increasing exposure.2 Fortunately, these rarely occur in the diagnostic imaging setting. Stochastic effects demonstrate a no-threshold model with a variable probability of their occurrence and no level below which exposure to ionising radiation is deemed completely safe.2 Greater exposure increases the likelihood of these effects occurring. Such effects include carcinogenesis, of which the risk has been recently quantified,3 and may occur years remote from the exposure.4
Worldwide, there is growing concern about radiation exposure. There is increasing concern regarding the issue of radiation dose and CT scanning and this issue is attracting increasing attention in the medical literature and also in the print and other news media.1 Patients with chronic illness have been demonstrated ‘at risk’ for high cumulative exposures to ionising radiation as a result of undergoing diagnostic imaging studies. In these populations, the imaging modality that contributed the majority of exposure was computed tomography.1
Cumulative radiation exposure in excess of 75mSv has been estimated to increase cancer mortality in the general population by 7.3%.4 This dose approximates to four or five CT thorax-abdomen-pelvis examinations. Concern regarding exposure to ionising radiation was, until recently, less likely to be considered a major issue for oncology patients than for patients of a similar age with benign disease.5 A retrospective review of cumulative radiation exposure from diagnostic imaging in oncology patients demonstrated that, during the five years after cancer diagnosis, 10-16% of cancer survivors received a five-year effective dose of 100mSv; in years six through 10 of follow-up, 3.8-7% received an effective dose in excess of 100mSv.6
Oncology patients form a large spectrum, with varying disease types, stage and treatment regimens. As a group, however, they are unique in the extent of reliance on imaging to assess response and disease-free survival. The detrimental effects of high cumulative exposures in these patients can be confounded by the already significant, albeit focussed, radiation doses incurred from radiotherapy. Many patients are also on immunomodulatory therapies, which lower the threshold at which detrimental effects are likely to occur.
With improvements and advances in both medical and surgical treatments for malignancies, cancer survivorship is increasing, often beyond that catered for in current follow-up guidelines. Cure can often be achieved. In this context, the potential hazard of ionising radiation that is associated with diagnostic imaging in patients with malignant disease is an increasingly important issue.
CT follow-up is the mainstay of many cancers, core to the recommendations of guidelines such as those of National Comprehensive Cancer Network (NCCN) and National Institute for Clinical Excellence (NICE). While such exposures are justified, they should still be performed with care. The long-term risks of radiation exposure in cancer survivors must be addressed.
Radiation exposure and associated risks
Cumulative radiation exposure is controlled by two variables: the number of individual imaging studies and the time period over which these scans are performed. Oncology is a guideline-directed specialty with specific and tailored follow-up protocols for different types and subtypes of cancer, dependent on the employed treatment regimen, stage at diagnosis, etc. These guidelines do not always consider the impact of cumulative radiation dose on these patients in this era of improved cancer survivorship.5 Many recommend CT follow-up as a mainstay with relatively short inter-scan intervals and, often, multiphasic studies, with less reliance on non-radiation employing modalities, such as MRI and ultrasound. Frequently, whole body CT surveillance is performed routinely, even after prolonged periods of disease-free survival in patients with restricted disease.
Children and young adults with cancer
Children, adolescents and young adults with cancer form a particular ‘at risk’ group within the oncology cohort. Patients diagnosed with malignancy at a young age are more likely to receive higher lifetime cumulative radiation exposure.3,7,8 Children receive a greater organ dose than adults from an individual scan and have a greater inherent sensitivity to development of radiation-induced cancer than adults.7 The potential for the induction of a second malignancy in this population is significant,3 considering that many of the childhood cancers are potentially curable.
For patients less than 10 years old, it is estimated that in the 10 years following the first scan, one excess case of leukaemia and one excess brain tumour occurs per 10,000 head CTs.3 It is estimated that for every 6,000 routine abdominal and head CT examinations performed in children under the age of 15, five cancers directly attributable to ionising radiation will occur.7 These adverse effects have a late expression, often decades after the actual exposure has occurred. Thus, care needs to be taken in the imaging of all oncology patients, but particularly in those where the initial diagnosis was made as a child.
Current CT techniques for dose reduction
The use of CT, particularly complex multi-phase studies, should be justified and optimised for the individual patient. Where available and appropriate, consideration should be given to non-radiation employing modalities such as MRI and ultrasound.
Where CT and PET CT are indicated, they should be used judiciously with limitation of the scanned field to the area of interest, where possible, and avoidance of multi-phasic studies, where appropriate. Inter-scan intervals should be tailored to the specific disease type and/or treatment regimen.
Simple measures, such as avoidance of duplicate imaging (eg. CT TAP in a patient who has had a recent PET CT), allow potential for further dose reduction without loss of diagnostic information. Multidisciplinary conferences have a role to play in the avoidance of excessive radiation exposure.
Inter-individual patient factors should also be taken into account and tailored diagnostics/follow-up have a role to play in the future of oncological imaging.9 In much the same way as therapy is tailored to an individual patient based on their disease type and extent, as well as other patient factors such as comorbidities, the imaging performed for the diagnosis and follow-up of these patients can also be tailored on the basis of tumour type and stage, history of illness (including response to therapy) and cumulative radiation exposure from all sources.
For example, patients with markedly reduced life expectancies are unlikely to manifest the deleterious effects of radiation exposure due to the long latency period and, thus, criteria for imaging in these patients should not necessarily be the same for those with normal life expectancies or curable disease.10 Conversely, in children and young adults, utmost attention should be given to potential reduction of cumulative radiation exposure.
Novel technologies
CT remains the mainstay of oncology imaging, with PET CT occupying an expanding role. Emerging and novel CT technologies have potential to facilitate acquisition of diagnostic quality studies at the lowest possible radiation dose.11 While low dose acquisitions may result in increased image noise in may instances, in specific cancers or after defined durations of imaging follow-up, image noise with disease-specific low dose CT protocols may be acceptable especially when high contrast resolution is not mandatory.
Automatic tube current modulation
Fixed tube kilovoltage and amperage settings have traditionally been used in CT imaging, meaning inefficient radiation exposure, where less attenuating body areas such as the thorax receive the same exposure as more attenuating regions such as the pelvis.
Automatic tube current modulation (ATCM) adjusts tube current during a CT scan depending on X-ray attenuation in that anatomic location on the topogram, and tailors the current output of the CT tube to patient size and shape.12 This method ensures that thicker regions of the body are imaged using higher tube currents than thinner, less attenuating areas. A reduction in tube-current time product can be achieved in 87% of examinations using ATCM, with an average tube-current time product reduction of 32%.13
Noise reduction filters
Noise reduction filter (NRF) software applications are used to post-process CT images acquired at significantly reduced radiation dose, improving their diagnostic quality by reducing image noise.14,15
CT reconstruction algorithms
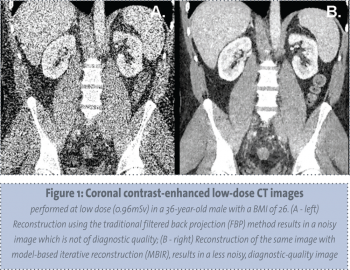
The most significant recent advances in dose-reduction technology comprise novel CT reconstruction algorithms. Filtered back projection (FBP), the conventional CT data reconstruction algorithm, has limited use with low-dose protocols given the propensity for increased imaged noise.16 Iterative reconstruction is a novel reconstruction algorithm in CT which, when used in place of filtered back projection, improved noise and spatial qualities within the image.17 This allows diagnostic quality images to be obtained at significantly reduced radiation doses without the prohibitive noise that would otherwise be present.18
The current, more widely used type of iterative reconstruction is a hybrid with filtered back projection (Adaptive Statistical Iterative Reconstruction (ASIR); General Electric Healthcare). Various modifications of iterative reconstruction are being developed and refined by different CT manufacturers including: Iterative Reconstruction in Image Space (IRIS) (Siemens Healthcare), Adaptive Iterative Dose Reduction (AIDR) (Toshiba Medical Systems), and iDose (Phillips Healthcare).
The next generation of iterative reconstruction is model-based iterative reconstruction (MBIR) which will allow for further dose reduction, beyond that achieved with ASIR, and offers the potential to further enhance image quality at even lower radiation doses than ASIR.19 MBIR is time consuming with a resultant requirement for extensive computing power, though this technology is being continually refined. MBIR has recently become available in clinical practice (see Figure 1).
 (click to enlarge)
(click to enlarge)
