CANCER
Obesity, inflammation and cancer
Investigation of anti-inflammatory agents and further understanding of the link between obesity, inflammation and cancer may provide new avenues for the development of anti-cancer treatments
December 1, 2012
-
Cancers arise as a result of acquired changes in the DNA of cancer cells. Numerous anomalies develop with time and these mutations are positively selected for the micro-environment of the tissue.
Obesity is a state of chronic inflammation
Obesity is characterised by increased storage of fatty acids in an expanded adipose tissue mass.1 Increased adipose tissue mass, especially visceral adipose tissue, is associated with insulin resistance, hyperglycaemia, dyslipidaemia, hypertension and other components of the metabolic syndrome.2 Adipocytes from obese subjects exhibit an altered endocrine function and secretory profile leading to an increased release of pro-inflammatory molecules resulting in a chronic low-grade inflammatory state that has been linked to the development of chronic diseases, including cancer.2
Despite an expanding body of epidemiological evidence in support of the link between obesity and cancer, the underlying molecular mechanisms responsible are poorly characterised. Excess adipose tissue results in elevated levels of pro-inflammatory adipokines, resulting in an imbalance between increased inflammatory stimuli and decreased anti-inflammatory mechanism leading to persistent low-grade inflammation.3-5
Adipose tissue is principally deposited in two compartments – subcutaneously and centrally. It is thought that centrally deposited, or visceral, fat is more metabolically active than peripheral subcutaneous fat.1,6,7
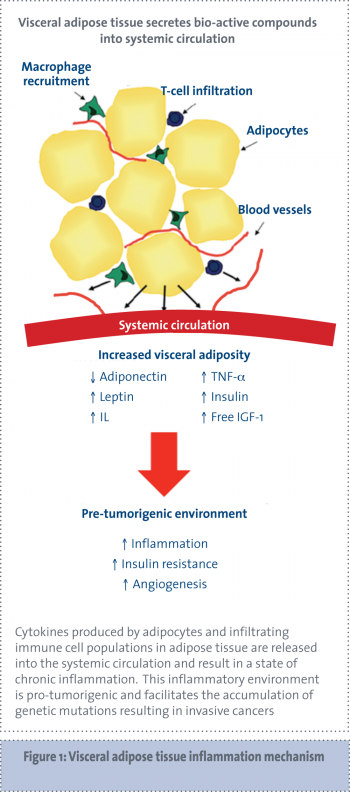
Visceral adipose tissue is largely comprised of omental adipose tissue but also includes other intra-abdominal fat sources, such as mesenteric fat. Visceral adipose tissue secretes a number of adipokines and cytokines leading to a pro-inflammatory, procoagulant and insulin-resistant state, collectively known as the metabolic syndrome.8
The importance of adipose tissue location in terms of dysmetabolism risk is evident as central obesity is more strongly associated with increased risk of insulin resistance, the metabolic syndrome and cardiovascular diseases than BMI alone.9 For any given amount of total body fat, the subgroup of individuals with excess visceral fat (versus subcutaneous fat) is at higher risk of developing insulin resistance10 and the features of the metabolic syndrome.11
Visceral fat remains more strongly associated with an adverse metabolic risk profile even after accounting for the contribution of other standard anthropometric indices.12 These systemic effects exerted by visceral adiposity are putatively involved in cancer biology13 and are the focus of current research.
Inflammation is a cancer hallmark
Hanahan and Weinberg proposed six hallmarks that define properties that tumours acquire in order to maintain a malignant phenotype, including tissue invasion and metastasis, evasion of apoptosis, sustained angiogenesis, insensitivity to growth inhibitors, limitless replicative potential and self-sufficiency in growth signals.14 Mantovani proposed the addition of a seventh property: an inflammatory micro-environment.15
Adipose tissue, through the systemic alterations associated with obesity, may support the development of malignant potential in susceptible cells through supporting the other malignant processes within cancer cells, such as invasion and metastases, evasion of apoptosis, promotion of angiogenesis and systemic inflammation. Inflammation provides the selective pressure that may drive the accumulation of mutations and result in poor immune surveillance of tumours once they develop.
Insulin resistance and obesity
Obesity is associated with an increased risk of developing insulin resistance and type 2 diabetes. Nutritionally-induced insulin resistance develops as a metabolic adaptation to increased circulating levels of free fatty acids (FFAs), which are constantly released from adipose tissue, especially from visceral fat stores.16
Increased FFA levels force liver muscle and other tissues to shift towards increased storage and oxidation of fats for their energy production.17 The compensatory effect is a reduced capacity of these tissues to absorb, store and metabolise glucose. Insulin resistance and type 2 diabetes have been recently recognised as intimately associated with the presence of systemic inflammation. There are increased levels of markers and mediators of inflammation including acute phage reactants, such as fibrinogen, C-reactive protein, IL-6, plasminogen activator inhibitor-1 (PAI-1) and white cell count in patients with newly diagnosed type 2 diabetes.18
Increased insulin in the insulin-resistant state is a mitogen
Increased numbers of adipocytes releasing FFAs are not alone sufficient for the development of insulin resistance. In addition to increased FFA levels, high concentrations of cytokines produced by adipose tissue, such as TNF-alpha, interleukin (IL) 6 and IL-1beta, and low concentrations of adiponectin are required for deleterious effects on glucose homeostasis.19
The cellular and molecular mechanisms leading to insulin resistance include a reduction in cellular insulin-receptor levels and reduced responsiveness of some intracellular transduction pathways mediating the effects of insulin binding to its receptor.20 Insulin resistance leads to increased insulin production and insulin can act as a mitogen and has been associated with several cancers.21-23
The tumorigenic effects of insulin could be directly mediated by insulin receptors in the pre-neoplastic target cells, or might be due to related changes in endogenous hormone metabolism, secondary to hyperinsulinaemia 16
Adipose tissue in obese individuals is infiltrated by immune cells
Cytokines secreted by adipose tissue include the following pro-inflammatory cytokines: TNF-alpha, IL-6, IL-8, IL-10, IL-1 receptor agonist (IL-1Ra), macrophage inflammatory protein 1 (MIP-1) and monocyte chemo-attractant protein 1 (MCP-1). The increased size and number of adipocytes in adipose tissue results in areas of hypoxia within the tissue. This hypoxia induces the secretion of inflammatory factors in order to promote angiogenesis.24-26
Inflammatory cytokines attract infiltrating immune cells, which in turn produce more inflammatory cytokines. In fact, the overall level of adipokine production from adipose tissue is strongly influenced by the degree of immune cell infiltration present in adipose tissue.6,27-29
Adipose tissue in obese people is infiltrated with macrophages and the number of macrophages correlates with the degree of adiposity (see Figure 1).30