CANCER
Advanced Breast Cancer Second International Consensus Conference (ABC2) highlights
Treatment advances, and unaddressed psychological and quality of life needs, featured at the recent Advanced Breast Cancer Conference
February 18, 2014
-
“First that patients must be at the heart of everything we do, not just as beneficiaries of care but as participants in shared decision making. As patients there should be no decision about us without us.” Andrew Langsley, UK Secretary of State for Health, 2011.1
Breast cancer is one of the most common cancers worldwide with approximately 1.4 million women diagnosed each year. Despite declining mortality rates since the early 1990s, largely attributable to improvements in adjuvant therapy for early disease,2 breast cancer remains the leading cause of cancer death in women in the developed and developing world. One 10th of patients have metastatic disease at presentation and 30% of women with early breast cancer will develop advanced disease.3 Of these patients, 10% will live with metastatic disease for 10 years or more, with the majority surviving for several years.
A lack of patient registers that measure relapse means that the true extent of advanced breast cancer (ABC) in many countries is unknown. Although improvements in therapy have changed the trajectory of ABC from acutely life-threatening to a chronic disease for many patients, it remains without cure and accounts for over half a million deaths worldwide a year.
There is a growing body of evidence highlighting significant unaddressed psychological and quality of life needs among patients living with ABC. Traditionally, resourcing patients with breast cancer has focused on those with newly diagnosed and potentially curable disease. Many patients contrast the abundant support received when they are diagnosed to the dearth of support that is present at relapse, leaving patients feeling isolated and abandoned.
ABC consensus conference
The ABC consensus meeting stems from the establishment by the European School of Oncology (ESO), in 2004, of the Advanced Breast Cancer Task Force, to develop comprehensive, international guidelines for the management of the disease.
The first Advanced Breast Cancer Consensus Conference (ABC1), held in Lisbon, Portugal, in November 2011, facilitated the development of the first international guidelines specific to advanced breast cancer.4 These guidelines were updated by an expert panel at the second consensus conference (ABC2) in November 2013. The expert panel of 40 comprised world leaders in oncology and palliative care, ABC patients, advocates, specialised nurses and psycho-oncologists.
The consensus panel ABC2
The updated guidelines will be developed in co-operation with the ESO and the European Society for Medical Oncology (ESMO) and endorsed by the European Society of Breast Cancer Specialists (EUSOMA), Federación Latinoamericana de Mastologia (FLAM) and the Senologic International Society (SIS). Publication of the ABC2 guidelines is expected early this year.
Highlights from ABC2
Patient experience and advocacy
Unique to this event is the strong presence and central role played by ABC patients and advocates. ABC2 saw the addition of a specific programme for breast cancer advocates co-ordinated by leading advocacy groups worldwide. An ABC Patient Advocacy Committee was established, involving 68 advocates, many with advanced breast cancer, from 45 organisations and representing 25 countries.
Keynote speaker Doris Fenech, an advanced breast cancer patient, nurse and advocate, delivered a powerful account of the physical and emotional needs of women with metastatic disease, as distinct from those with early-stage disease.
She highlighted the lack of clinician training in advanced disease management as well as a perceived lack of interest from clinicians to treat advanced breast cancer.
“For most healthcare professionals a diagnosis of metastatic breast cancer is the beginning of the end and frequently they are at a loss as what to say to us and how to help.
“These attitudes have contributed to misconceptions and marginalisation by the public, the media and healthcare professionals themselves,” said Ms Fenech.
This feeling of isolation is echoed in the final results of the Here & Now pan-European ABC patient and carer survey and ABC awareness consumer poll, both supported by Novartis Oncology. Completed by 158 ABC patients and 146 carers in nine countries, the Here & Now survey explores the psychological, social and economic impact of ABC on patients and their families.
In this survey, eight out of 10 patients said that quality of life is the biggest area for improvement in the provision of care. Depression was reported by 41%, with over 50% of patients experiencing worry. More than one third of patients reported loss of confidence and personal identity, and 38% expressed fear of the future. Half of all respondents confirmed that pain interferes with their daily life.
Also presented were results from the multinational online survey Count Us, Know Us, Join Us, which captured similarly high levels of distress. This was completed by 1,273 female patients with metastatic breast cancer from 12 countries. Of these women, 68% stated that ABC had a moderate or severe negative impact on their quality of life. Almost two thirds of respondents (64%) reported a significant negative effect on their emotional wellbeing, and 57% said it had a negative impact on the emotional health of their loved-ones. Despite this high level of unmet need, only 51% had discussed quality of life issues with their healthcare provider and less than 40% had discussed the impact of ABC on their emotional health.5
Improved understanding and awareness of the significant psychological morbidity associated with ABC is essential. Approximately 40% of patients with ABC suffer from depression and/or anxiety that merits intervention. Lesley Fellowfield, professor of psycho-oncology, University of Sussex, highlighted the need for improvement in the current use of psychosocial resources, with a more targeted approach using systematic screening in order to identify those who require specialist referral.
Identified healthcare system needs
In keeping with the strong multidisciplinary ethos established at ABC1, the updated recommendations will reflect an urgent need for specialist oncology nurses for advanced disease. A consistent theme put forward by patients was the ongoing need for continuity of care and improved communication; balancing hope and reality.
Advocate groups emphasised the importance of access to relevant clinical trials for ABC patients, the need for standardised and affordable treatment worldwide, and equal access to appropriate palliative care from diagnosis.
The establishment of patient registers and tumour boards should be prioritised. The value of information sharing and improved interaction between societies and support groups, both regionally and globally, rather than duplication of effort was also highlighted. A position paper by advocates at ABC2 is expected to be published in early 2014.
Major therapeutic advances since ABC1
HER2-positive breast cancer
The development of highly effective therapies directed at the human epidermal growth factor receptor 2 (HER2) has dramatically changed the therapeutic landscape for HER2-positive breast cancer of all stages over the past decade.
Approximately 20-30% of breast cancer patients have tumours that over-express HER2, associated with an aggressive tumour phenotype and poorer prognosis. The recombinant monoclonal antibody, trastuzumab has shown significant clinical benefit in early stage and metastatic HER2-positive disease. The development of eventual trastuzumab resistance has led to the development of alternative HER2-directed therapies.
HER2, a valid target following progression on trastuzumab
Since ABC1, major developments include the approval of monoclonal antibody pertuzumab for advanced HER2-positive breast cancer. Pertuzumab and trastuzumab bind to different regions on the HER2 receptor and can have synergistic activity. The combination of pertuzumab plus trastuzumab plus docetaxel, compared with placebo plus trastuzumab plus docetaxel, as first-line treatment for HER2-positive metastatic breast cancer, significantly prolonged progression-free survival and overall survival, achieving an overall response rate of 80% with no increase in cardiac toxicity.6,7
Sequence of HER2-directed therapy
Pertuzumab in combination with trastuzumab is now considered the new standard of care in the first-line setting. Studies to establish the most suitable chemotherapy partners are ongoing, the current recommendation being paclitaxel or docetaxel while further data is awaited.8 Although only licensed in the first-line setting, the availability of pertuzumab remains limited and, as yet, it is not available in Ireland.
Phase II data investigating combination pertuzumab and trastuzumab for patients with metastatic breast cancer with progression on trastuzumab has demonstrated an overall response rate of 25% and a median progression-free survival of 5.5 months, suggesting a rationale for its use beyond first-line.9 The question as to whether pertuzumab should be given beyond progression with rotation of chemotherapy, like trastuzumab, is yet to be addressed in clinical trials.
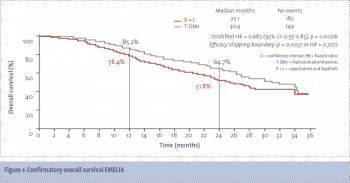
The preferred second-line agent is the antibody-drug conjugate trastuzumab emtansine (T-DM1). T-DM1 has been shown to be superior to capecitabine and lapatinib with improved overall survival, improved progression-free survival and a superior toxicity profile (see Figure 1).10