CANCER
PAIN
Cancer pain management at the end of life
Management of pain in the final 72 hours of life
November 8, 2013
-
Pain is described by the International Association for the Study of Pain (IASP) as: “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 Special consideration needs to be given to the management of pain at the end of life.
This article will concentrate on the management of pain in the final 72 hours of life and is intended as a practical tool to aid doctors and other healthcare professionals manage pain in the dying patient. If pain is not optimally controlled it will contribute to the suffering of the dying patient and will heighten the distress of the family.
The final days
It is difficult to be accurate concerning prognostication of diseases. Generally only estimates in terms of days, weeks or months can be given to patients and their families. The clearest signs of approaching death are picked up by the day-to-day assessment of deterioration. Such signs include profound tiredness and weakness, reduced intake of food and fluids, drowsy or reduced cognition, gaunt appearance and difficulty swallowing medicine.2 If such symptoms develop suddenly or unexpectedly it is important to exclude a reversible cause of the deterioration such as infection, hypercalcaemia or side-effects of medications.
An important cause to consider is opioid toxicity. Patients can develop opioid toxicity during titration of opioids, however, toxicity can occur in patients who are stable on long-term opioids.
Opioid toxicity is usually associated with delirium with myoclonus, vivid dreams and hallucinations.3 If these are not present other causes of delirium should be considered. It is also important to acknowledge that a patient may continue to deteriorate and die despite the treatment of infection or biochemical abnormalities. Once it is clinically considered that a patient is beginning to die the focus of the patient’s management changes to optimising comfort. Unnecessary procedures, such as phlebotomy and vital sign monitoring, should be discontinued.
In order to decrease the burden of taking tablets, medications that are no longer essential and do not provide benefit in terms of patient comfort should be rationalised.
Communication
It is essential for accurate information to be communicated clearly to the patient and family. Certain changes in the patient’s care will be taking place that the patient and family will be unfamiliar with, such as the discontinuation of regular medications and vital sign monitoring. It is important to anticipate patients’ and families’ fears and pre-emptively manage these concerns where possible with good communication.
Medications may need to be given parenterally, such as in a continuous subcutaneous infusion (CSI), if a patient is too weak to swallow. However, this may raise some concerns for the patient and family that it could cause or hasten death. If expressed, these fears should be explored, and the reason for using a CSI should be clearly communicated.
It is important for the family to be aware that it is the progressive nature of the underlying condition that will lead to the patient’s death and not the use of a CSI, which is simply to deliver medication that can no longer be administered via the oral route and to prevent frequent parenteral medication usage.
Pain assessment in the dying patient
A number of symptoms can develop during the dying phase such as pain, excessive respiratory secretions, and changes in breathing patterns. Due to a patient’s reduced consciousness they may not be able to report pain, and as such, pain is best monitored by observing a patient’s behaviours for non-verbal clues. These include facial expressions such as grimacing and frowning, vocalisations such as groaning, and body language such as a tense posture.4
As well as consideration being given to the assessment of pain in the dying patient, the choice of analgesic also requires special attention.
The World Health Organisation (WHO) guide to the management of cancer pain is based on a three-step analgesic ladder. Step one analgesics include paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs) and aspirin. Step two analgesics include codeine and tramadol. Morphine sulphate, oxycodone, hydromorphone, buprenorphine and fentanyl are examples of ‘strong opioids’ that are commonly prescribed for use at step three of the WHO ladder.
Some step one analgesics, such as diclofenac and paracetamol, have a role in the pain management of the dying. Paracetamol can be administered intravenously and per rectum. Diclofenac is available in Ireland as an injection. It is only licensed for intravenous and intramuscular use; however, anecdotal evidence in the palliative care setting supports its routine use subcutaneously. It is typically given as a continuous subcutaneous infusion at a dose of up to 150mg/24 hours.5,6 Diclofenac can also be administered per rectum.
Step two analgesics, weak opioids, have no role in pain management in the dying, as these are not administered using a continuous subcutaneous infusion. The WHO step three analgesics, strong opioids, are the mainstay of pain management in the dying because of their ease of use in CSIs.
When patients are dying they may not have any pain and may not be prescribed any regular analgesic. In this scenario it is sufficient to prescribe analgesia on a pro re nata (prn) basis that can be administered if the patient develops pain. If frequent doses are required it is less burdensome for a patient to have a continuous subcutaneous infusion to deliver the medication than regular bolus injections. A continuous subcutaneous infusion of opioids is easy to administer and can be given at home. Many opioids can be used alone or in combination with other medications in a CSI.
If a patient is currently taking regular weak opioids for pain management and the oral route is no longer available an appropriate dose of a strong opioid should be administered subcutaneously.
Conversion tables are available of opioid equivalencies. These conversions are a guide only and doses may vary for individual patients.
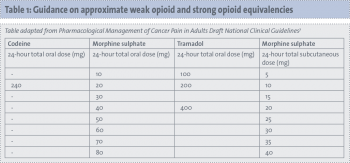 (click to enlarge)
(click to enlarge)

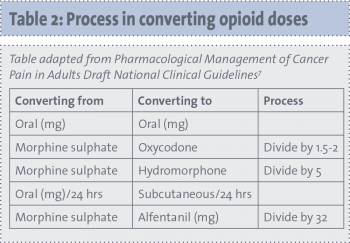 (click to enlarge)
(click to enlarge)