CANCER
Exercise as a treatment to improve prostate cancer morbidity and mortality
An exploration on the effect of exercise on prostate cancer morbidity and mortality
May 11, 2016
-
Exercise is emerging as a successful non-pharmacological treatment to achieve significant improvements in prostate cancer (PCa) morbidity and mortality. A landmark paper in this area reported that men with PCa who exercised vigorously (eg. cycling, tennis, jogging, swimming) for three or more hours per week had a 61% lower risk of death from PCa compared to men who exercised vigorously for less than one hour per week.1 Multiple epidemiological studies have suggested that obesity is also associated with increased PCa mortality2 and may represent a key component of the hypothesised mechanisms underpinning the relationship between physical activity and PCa outcome.
Androgen deprivation therapy
Patients with PCa, especially those on androgen deprivation therapy (ADT), may experience many debilitating symptoms from treatment, including changes in body composition. Prospective evaluation of 79 men during initial androgen deprivation therapy from baseline to 12 months showed ADT increased weight (1.8% SE 0.5%) and fat mass (11.0% SE 1.7%) and decreased lean mass (3.8% SE 0.6%) in men with nonmetastatic Pca.3,4 The loss of lean mass and increase in fat mass associated with ADT has major implications for the functional independence and comorbid disease risk of patients living with PCa. Exercise may provide a reasonable strategy to counteract the many adverse symptoms of PCa disease and treatments such as ADT. Exercise programmes are efficacious in improving body composition, exercise capacity, physical function and quality of life in patients with Pca.5 Engaging patients in regular exercise is essential to improve patient outcomes post-prostate cancer diagnosis and optimise health during treatment.6
Exercise programme
The American Cancer Society and the Prostate Cancer Foundation recommend that all patients with PCa adopt a regular exercise programme consisting of aerobic and resistance exercise.7,8 Treating clinicians should play a role in directly advocating the benefits of exercise to men with PCa and leading referral to a formal exercise programme when indicated.5 Moderate-to-vigorous intensity aerobic exercise is most often prescribed (55-100% maximum heart rate) while moderate to high intensity resistance exercise is recommended (60-80% 1 repetition maximum).9,10,11
Several exercise interventions have shown many positive physiological benefits for patients living with PCa.12 A three-month aerobic and resistance exercise programme preserved appendicular lean mass in men receiving ADT when compared with a usual care group, who lost ≈ 0.6kg (adjusted between group difference of 0.4kg, 95% CI 0.1 to 0.7). Furthermore, reductions were observed in fat mass (-1.4kg 95% CI -2.3 to 0.6), trunk fat mass (-0.9kg 95% CI -1.5kg to 0.2kg) and percentage fat (-1.3% 95% CI -1.7kg to 0.8) in the exercise group when compared with usual care (13). A three-armed randomised controlled trial (RCT) comparing six months of both resistance training (n = 40) and aerobic training (n = 40) to usual care (n = 40) in men receiving radiotherapy for PCa, found that when compared to aerobic training, resistance training provided additional longer term benefits in reducing body fat percentage (-0.16% and -1.5% respectively when compared to usual care). In comparison, physical fitness was maintained over the entire study period in both the resistance training (0.14ml/kg/min [95% CI -0.9 to -1.2 units]) and in the aerobic training groups (0.04ml/kg/min [95% CI -0.98 to -1.1 units]). In contrast, patients receiving usual care experienced a significant decline in aerobic fitness over 24 weeks of -1.4ml/kg/min (95% CI -2.4 to -0.4) from a baseline fitness of 27.6ml/kg/min.11 Trials examining these outcomes are ongoing.
Metastatic bone disease
The clinical course of metastatic bone disease in prostate cancer survivors is relatively long, with a five-year survival rate of approximately 30%.14,15 Bone is the main metastatic site in about 80% of patients with PCa.16 Patients with bone metastasis are at risk of muscle atrophy and increased likelihood of skeletal complications;17 however both aerobic and resistance exercise were shown to be safe and feasible in this population.10,13 No deaths attributable to exercise interventions are detailed across four trials prescribing both aerobic and resistance exercise training to patients with advanced PCa. 10, 11, 18, 19 Other serious adverse events (eg. myocardial infarction) are equivalent to control participants.5
As in patients with primary PCa, exercise programmes for patients living with advanced PCa should be individually tailored with consideration of the individual’s physical capabilities and limitations.20 Patients with advanced PCa should be taught skills on monitoring exercise intensity eg. using the Borg scale, and correct technique for resistance exercises.10
When disease burden is high, resistance programmes may select specific exercises based on the location of bone metastasis which ensure that affected regions are not targeted and mechanical force is minimised. Resistance exercise programmes adapted in this way have shown to be protective against muscle atrophy. A RCT involving a 12-week resistance programme for patients with advanced PCa resulted in favourable changes in whole body (3%) and appendicular (4%) lean mass in the exercise group when compared with the usual care group.19 This results in considerable benefits by ameliorating metabolic disorders through improved insulin resistance and glucose homeostasis.21 As evidence grows showing the important role exercise plays in moderating body composition in men with PCa, attention is increasingly turning to the identification of molecular mechanisms connecting body composition and PCa.
The link between obesity and cancer
In 2012, a population based study estimated that a high body mass index (BMI) and obesity were attributed to 3.6% of all new cancer cases worldwide.22 While there are substantial arguments against a direct relationship between overall PCa risk and obesity,23 men who are classified as obese are more likely to suffer from aggressive disease,24 inferring a relationship between advanced PCa and obesity. In two large cohorts of patients with PCa enrolled in the Cancer Prevention Study in the US, CPS-I and CPS-II, high BMI was associated with poor overall survival or increased cancer related mortality.25 Physical activity has been associated with a decreased likelihood of diagnosis of advanced, metastatic PCa.26
Metabolic syndrome
Metabolic syndrome is defined as low level systemic inflammation and a group of factors, such as central adiposity, hypogonadism and hypertension, which together pose a greater risk for cardiovascular disease. Metabolic syndrome is linked with more aggressive, advanced PCa.27 In-vitro PCa cell line studies have demonstrated a direct link between adipocytes and changes in tumour cell proliferation and differentiation patterns.28 Adipocytes, or fat cells, secrete adipokines, some of which are associated with PCa. Anti-proliferative properties have been demonstrated in PCa cell line studies using adiponectin.29 In similar cell line studies, leptin was shown to enhance proliferation and to promote angiogenesis.30 These effects have also been demonstrated in PCa patients with a link between aggressive PCa and leptin levels reported.31 Systemic inflammation is recognised as a risk factor in many disease states, including cancer. Increased levels of pro-inflammatory cytokines such TNFa are thought to have a role to play in cancer progression.32 Physical activity holds significant potential to reduce the risk of systemic inflammation and may play a role in improving quality of life.33
Natural killer cells
Exercise interventions in cancer survivors have demonstrated an increased immune response and an increase in natural killer cell numbers post physical activity. Natural killer cells are paramount to the body’s innate immune response and play a role in the detection and destruction of atypical cells present in the bloodstream. Decreased levels of natural killer cells have been correlated with an increased risk of cancer.34 Circulating tumour cells (CTCa) are tumour cells which are thought to have shed off of a primary tumour or metastatic lesions and entered the bloodstream before extravasating to distant sites.35 It has been postulated that the extravasation of CTCs is aided by a method known as platelet cloaking.36 Platelet cloaking is thought to occur when activated platelets surround or cloak tumour cells and prevent them from being detected by natural killer cells thus contributing to the metastatic spread.37 In-vitro models using ovarian cancer cell lines have established that platelets aggregate to tumour cells and provide a protective pro-survival environment, leading to the hypothesis that they aid tumour cell metastasis.38
ExPeCT clinical trial
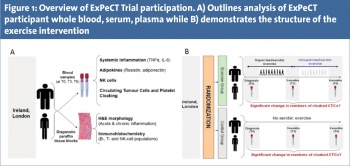
With increasing evidence examining the benefits of physical activity in metastatic PCa patients, there is an emerging need for a comprehensive exercise intervention programme incorporating advanced patients. ExPeCT (exercise, prostate cancer and circulating tumour cells) is a multi-centre randomised clinical trial for men with metastatic PCa, funded by the World Cancer Research Foundation (Clinicaltrials.gov NLM Identifier: NCT02453139). Recruitment for ExPeCT is underway in five Irish sites in conjunction with the All-Ireland Co-operative Clinical Research Group (ICORG) and one UK site (St Guy’s Hospital, London). ExPeCT has a target accrual of 200 participants and once enrolled in the study, patients are randomised into an exercise or control group and followed for a total of six months. The exercise programme consists of three months supervised aerobic exercise, followed by a three month unsupervised home based regime. Aerobic exercise will be based on an increase in heart rate and a range of activities, such as walking and jogging, are utilised. Due to the presence of lower limb metastatic bone lesions in these participants, equipment such as upper limb ergometers are used to accommodate any discomfort caused by metastatic burden. Participants randomised to the control group continue their standard care.
All participants are followed at three time points; baseline (T0), three months (T3) and six months (T6) (see Figure 1). Whole blood, serum and plasma are analysed for a series of systemic inflammatory markers; CTC number and platelet cloaking. Results of participants with a BMI of > 25 will be compared to the results of participants with a BMI of < 25. The overall aim of the ExPeCT trial is to examine the relationship between PCa, exercise, obesity and systemic inflammation, and to improve the overall quality of life in men with advanced disease.
The ExPeCT trial lays the foundation for the forthcoming Movember Global Prostate Cancer, Exercise and Metabolic Health Initiative (GAP4) which aims to better understand PCa progression.34 This will be a large scale multi-centre exercise trial for men with advanced cancer looking at overall survival as an endpoint and with additional endpoints focused on the mechanisms of action of physical activity on the biology of advanced disease.
Men in the intervention group will be supported with personalised exercise regimes that are tailored to their specific needs and abilities. Patients will be recruited at many sites from different countries around the world including Ireland and, in a global collaborative effort, data and biological samples will be shared and studied across borders by leading scientists and clinicians. This global translational research initiative aims to better understand the biological mechanisms by which exercise effects PCa.
 (click to enlarge)
(click to enlarge)
