CANCER
Immunotherapy for advanced urothelial carcinoma
Urothelial carcinoma can occur at any site throughout the urinary tract
March 24, 2017
-
Urothelial carcinoma can occur at any site throughout the urinary tract, which is lined by the urothelium. This includes the renal pelvis, ureters, bladder and urethra. More than 90% of these tumours originate in the bladder, with a further 5-7% originating in the upper urinary tract at the renal pelvis or ureters.1
Irish statistics show that urothelial carcinoma is the fourth most common cancer in men, and the 12th most common cancer in women. The average number of new cases diagnosed in Ireland each year is 306 in men, 135 in women and overall, it accounts for approximately 200 deaths each year.2 Treatment options for advanced disease have historically been limited and, until recently, there have been few advances since the advent of platinum-based chemotherapy in the 1980s.
Intra-vesical immunotherapy with Bacillus Calmette-Guérin (BCG) was the earliest form of immunotherapy used in the treatment of bladder cancer. This is applied in localised non-muscle invasive disease and uses a live attenuated form of mycobacterium bovis to stimulate a local immune response, targeting the bacteria and bladder cancer cells.
Intravesical BCG has been shown to decrease rates of recurrence and progression of localised non-muscle invasive bladder cancer.3,4 This provides proof-of-principle that urothelial carcinoma is susceptible to immune-response modifying therapies.
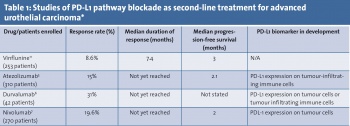
In metastatic disease, first-line treatment typically involves platinum-based chemotherapy using a cisplatin or carboplatin backbone. In the second-line setting however, there are few options and no accepted standard of care. Current options include vinflunine, taxanes and pemetrexed. Objective response rates are generally 10-20% and the treatments are often associated with considerable side-effects. Objective responses are usually short-lived.
Immune checkpoint inhibitors in the form of monoclonal antibodies targeting PD-1 or PD-L1 such as atezolizumab, nivolumab and pembrolizumab have previously shown impressive anti-tumour activity and prolongation of survival across multiple tumour types including melanoma, renal cell carcinoma and lung cancer. The evolution of these agents in the treatment of other malignancies lead to their exploration in urothelial carcinoma.
Atezolizumab
In May 2016, a study was published in The Lancet detailing a phase 2 trial which investigated the use of atezolizumab, a monoclonal antibody that binds to PD-L1, in patients with advanced urothelial carcinoma whose disease had progressed following previous platinum-based chemotherapy.5
A total of 315 patients were enrolled in the study, of whom 310 received atezolizumab. Results showed that treatment with atezolizumab was associated with an objective response rate of 15% and showed an improved duration of response and overall survival compared with historical comparators. Ongoing responses were recorded in 38 (84%) of 45 responders at a median follow-up of 11.7 months.
The most common treatment-related adverse events were fatigue (30%), nausea (14%) and anorexia (12%). Grade 3-4 toxicities occurred in 16% of patients, including fatigue (2%), pneumonitis (1%), and raised serum aspartate aminotransferase (1%). Overall, these results led to atezolizumab becoming an accepted second-line therapy in the treatment of urothelial carcinoma and it was FDA-approved for marketing in the US.
During the study, PD-L1 expression on tumour-infiltrating immune cells (ICs) was assessed by immunohistochemistry. Expression was categorised into different subsets: IC0 (< 1% expression), IC1 (≥ 1% expression), and IC2/3 (≥ 5% expression). Response was associated with increased PD-L1 expression, although there were responses seen in all subgroups: IC2/3: 26%, IC1: 11%, IC0: 8%, and 15% in all patients.
Durvalumab
Durvalumab is another PD-L1 antibody in development for urothelial cancer patients. A phase 1/2 study reported in the Journal of Clinical Oncology described its use in 61 patients with previously treated advanced urothelial cancer.6 Among 42 patients evaluable for response at the time of data cut-off, the objective response rate was 31%. Responses were durable and ongoing in 12 of 13 patients with durations ranging from four to 49 weeks at the time of data analysis and median duration of response was not reached.
Interestingly, the response rate was 46% among the 28 patients with PD-L1 positive tumours (defined as ≥ 25% staining in tumour cells or tumour infiltrating immune cells) compared with 0% in the 14 PD-L1-negative patients. These data suggest a robust biomarker of response for these patients, however the numbers reported are small and further investigation is required.
Among the 61 patients included in the safety analysis, the most common treatment-related adverse events were fatigue (13%), diarrhoea (10%) and anorexia (8%). Grade 3 treatment-related adverse events were seen in 4.9% of patients, these included acute kidney injury (biopsy-proven nephritis), an infusion-related reaction, and tumour flare (1.6% respectively).
Nivolumab
At ASCO 2016, the results of a phase 1/2 study of nivolumab, an anti PD-1 monoclonal antibody, in patients with previously treated metastatic urothelial cancer were presented (Checkmate 032). In that study, nivolumab demonstrated a durable objective response rate of 24%.7
In October 2016, the first results of the Checkmate 275 study were presented at ESMO 2016. This study is a large global, multi-centre, single-arm, phase 2 study, performed to confirm the anti-tumour activity of nivolumab in this patient population.8 Patients with metastatic or surgically unresectable urothelial cancer were enrolled. Patients had progressed on platinum-based therapy. A total of 270 patients were enrolled, 78% of whom were men in a heavily pre-treated population, and 84% of patients had visceral involvement.
Analysis revealed that, compared with a historical objective response rate of 10%, nivolumab was associated with an objective response rate of 19.6% and a complete response rate of 2.4% using the RECIST v1.1 criteria. The vast majority of responses were rapid, occurring at approximately eight weeks, coinciding with the first restaging evaluation. The median progression-free survival in all patients was two months. The median overall survival was 8.7 months.
This study showed that nivolumab demonstrated clinically meaningful efficacy and also has the potential to become a second-line standard of care for this disease. The most common grade 3-4 toxicities noted were fatigue (1.9%), diarrhoea (1.9%) and asthenia (1.5%). Individual tumour tissue was submitted to a central laboratory for PDL-1 testing. PDL-1 expression was not required for treatment, but all patients were required to have viable tumour tissue for PDL-1 testing.
Almost half (45.9%) of patients had PDL-1 tumour expression using a > 1% cut point and 30.7% of patients had PD-L1 tumour expression using a > 5% cut point. Objective response rates were 16.1% in patients with no PDL-1 tumour expression (< 1%), 23.8% in patients with ≥ 1% PDL-1 expression and 28.4% in patients with ≥ 5% PDL-1 expression
Pembrolizumab
Pembrolizumab, also an anti-PDL1 monoclonal antibody, was first approved for the treatment of metastatic melanoma in September 2014. In October 2016, preliminary results from the KEYNOTE-052 study were presented at ESMO. This open-label, multi-centre phase 2 study investigated the use of pembrolizumab as first-line therapy for locally advanced unresectable or metastatic urothelial cancer in cisplatin-ineligible patients.9
A total of 374 patients were enrolled in the study. The main reasons for cisplatin-ineligibility were an ECOG performance status of ≥ 2 (43%) and renal dysfunction (45%). Patients received 200mg pembrolizumab every three weeks until progressive disease, unacceptable toxicity or treatment to 24 months. An interim analysis of the first 100 patients was performed.
The overall objective response rate (per RECIST v1.1) was 24%, with 6% of patients achieving a complete response. Two-thirds (67%) of patients experienced a drug-related adverse event; most commonly fatigue (14%). The most common grade 3-4 toxicities were fatigue (4%), muscle spasms (2%) and diarrhoea (1%).
In late October 2016, it was announced that pembrolizumab met its primary endpoint of overall survival in the KEYNOTE-045 study and it was recommended that the trial be stopped early. KEYNOTE-045 was a randomised phase 3 study comparing pembrolizumab to investigator-choice chemotherapy (paclitaxel, docetaxel or vinflunine) in patients with locally advanced or metastatic urothelial carcinoma, which had progressed following prior platinum-based chemotherapy.10
The study randomised 542 patients and further outcome data are awaited. It is the first trial to directly show improved overall survival for immunotherapy compared with chemotherapy in this disease.
Conclusion
The advent of immune checkpoint blockade molecules has provided renal cell carcinoma patients with a novel class of therapeutics capable of inducing durable disease response and stability. However, a predictive biomarker or response capable of accurately identifying those patients who will respond remains illusive.
As documented in this article, there is an association between PD-L1 expression on tumour cells and immune cells with treatment response. However, the accuracy of this as a biomarker is suboptimal and its development is hampered by the multiple different assays and methods of measurement in use (see Table 1).
 (click to enlarge)
(click to enlarge)
