CANCER
Lung cancer – Is there a role for screening?
Despite considerable interest in developing a screening tool to detect early-stage lung cancer, lung cancer screening remains very much a controversial issue
May 1, 2013
-
Lung cancer is the most common cancer worldwide.1 It is also the leading cause of cancer mortality in Ireland, representing approximately 20% of all deaths due to cancer.2 Despite advances in therapy, five-year survival rates average approximately 16% for all patients with lung cancer.3 These poor survival figures, coupled with the success of screening to improve the outcomes in cervical and breast cancer, have been the impetus for studies to develop an effective lung cancer screening test. Despite considerable interest in developing a screening tool to detect early-stage lung cancer, lung cancer screening remains very much a controversial issue.3
Cancer screening
The goal of cancer screening is to identify asymptomatic patients with unrecognised symptoms and to identify those at increased risk. The concept of screening is based on the assumption that identifying and treating cancer in asymptomatic individuals will prevent cancer or improve survival. This assumes that there is a treatable phase of pre-cancer or cancer that, if left untreated, would progress. It also assumes that early treatment improves outcome compared with late treatment.
The important outcome for cancer screening is its impact on overall mortality. Therefore, screening should benefit the individual by increasing life expectancy, as well as increasing quality of life.
In the ideal screening, the rate of false-positive results (ie. screening that shows there is cancer present when in fact there is not) should be low to prevent unnecessary testing. The large fraction of the population should not be harmed (ie. low risk) and the screening test should not be so expensive that it places an onerous burden on the healthcare system.3 Thus, the ideal screening should:
- Improve outcome
- Be scientifically validated (eg. have acceptable sensitivity and specificity)
- Be low risk, reproducible, accessible and cost-effective.3
Lung cancer screening
Currently, most lung cancers are diagnosed clinically when patients present with symptoms, with approximately 75% of patients presenting with advanced local or metastatic disease that is not amenable to cure.4 Screening for lung cancer has the potential to identify the disease in the earlier stages of development and to reduce the risk of death.
Current available options for lung cancer screening include sputum cytology, chest radiograph (CXR) and low-dose computed tomography (LDCT) scanning.
Sputum cytology and chest x-ray
Several early controlled trials of sputum cytology and CXR screening for lung cancer have been performed. While these studies have shown that early detection of lung cancer is possible with such initiatives, there was no reduction in the number of advanced lung cancers detected or in the number of lung cancer deaths.5-12
Low-dose CT scan
Renewed enthusiasm for lung cancer screening came with the advent of low-dose CT (LDCT) imaging, which has the ability to detect smaller tumours than on CXR. Key questions in relation to screening for lung cancer with LDCT include:
- What are the potential benefits and potential harms of screening individuals at increased risk of developing lung cancer using LDCT?
- Which groups are most likely to benefit or not benefit from screening?
- In what setting is screening likely to be effective?
To establish the benefit of LDCT in lung cancer screening, the National Lung Screening Trial (NLST) in the US enrolled current or former heavy-smokers and compared LDCT with standard CXR. Primary results have been published showing that participants who received LDCT scans had a 20% lower risk of dying from lung cancer than participants who received standard CXR. However, there was a high rate of false-positive results.13
It should be noted that this population of smokers at high-risk for lung cancer was a highly motivated and primarily urban group, screened at major medical centres. These results alone, therefore, may not accurately predict the effects of recommending LDCT scanning for other populations.
Potential harm
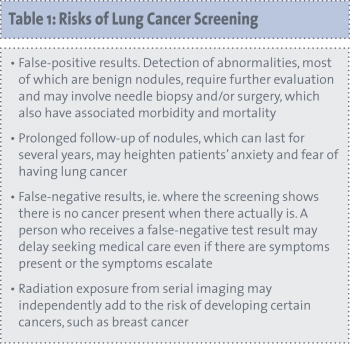
In theory, screening for lung cancer has the potential to reduce mortality and morbidity. There are potential harms, however, attached to screening as well as potential benefits. These risks need to be appreciated in order to determine the benefits (see Table 1).
 (click to enlarge)
(click to enlarge)
