GENERAL MEDICINE
Management strategies for saddle pulmonary embolism
A rare case of SPE where initial anticoagulation therapy showed limited improvement, however systemic thrombolysis therapy resulted in significant recovery for the patient
April 1, 2024
-
Saddle pulmonary embolism (SPE) is a rare type of acute pulmonary embolism (PE) that can lead to sudden haemodynamic collapse and death. It is defined radiologically as a thrombus spanning the pulmonary artery trunk and can be mistaken for massive PE, which is defined on the basis of haemodynamic instability.
Here, we present a case of SPE in a tertiary care setting, providing insights into its clinical presentation and management. The patient, a woman in her 70s, presented with abrupt-onset dyspnoea, chest tightness and dizziness. Elevated D-dimers prompted a CT pulmonary angiography, revealing a saddle thrombus with bilateral emboli. Although initial anticoagulation therapy showed limited improvement, thrombolysis resulted in significant recovery. The patient was subsequently transitioned to a direct oral anticoagulant (DOAC) and discharged with plans for ongoing monitoring. This case highlights the complexities involved in successful management strategies for SPE, offering valuable insights for clinicians.
Acute pulmonary embolism
Acute pulmonary embolism (PE) poses a potential threat to life and ranks as the third most common cause of cardiovascular death, following ischaemic heart disease and stroke.1,2,3,4 It represents the most severe manifestation of venous thromboembolism (VTE), carrying substantial risks of morbidity and mortality. When left untreated, the mortality rate for acute PE can reach as high as 30%. However, with timely diagnosis and appropriate treatment, the death rate for diagnosed and treated PE is approximately 8%.5
Saddle pulmonary embolism (PE) is a radiological term denoting a large clot that spans the bifurcation of the pulmonary artery trunk, often extending into bilateral main pulmonary arteries.6 It occurs in 2.6% to 5.4% of patients with PE.7,8 The presence of a large central thrombus in saddle PE may cause concern among clinicians, leading them to categorise it as massive PE and admit patients to the intensive care unit (ICU).
However, it is important to note that ‘massive PE’ is a haemodynamic definition, indicating any PE accompanied by shock and haemodynamic collapse.9 Therefore, it is inaccurate to label patients with saddle PE as having massive PE, as there are instances where these patients present with stable haemodynamic values and a relatively benign clinical profile. Saddle PE exhibits diverse characteristics with variable clinical features and outcomes. Limited data are available to guide clinicians in the general management of saddle PE. Some small studies have indicated that saddle PE does not necessarily result in an unfavourable clinical outcome, with mortality rates ranging from 4.5% to 16%.8,10
Case presentation
A woman in her 70s presented to the emergency department reporting shortness of breath. In the previous two days she began experiencing a gradual onset of shortness of breath, even at rest, which exacerbated with minimal exertion. She also reported sensations of chest tightness and dizziness. However, she denied experiencing chest pain, orthopnoea, proximal nocturnal dyspnoea, wheezing, ankle swelling, cough or the production of phlegm. Notably, she had recently been discharged from the hospital after being treated for a lower respiratory tract infection, and her recovery was progressing well, with almost complete resolution of infectious symptoms.
Her medical history included well-controlled type 2 diabetes and hypertension. She reported no recent surgeries. Regular medications comprised oral hypoglycaemic (metformin), an angiotensin receptor blocker (losartan) and aspirin (75mg OD). She denied any family history of clotting disorders. Additionally, she was a non-smoker and non-drinker, and she maintained complete independence in terms of mobility.
During examination in the ED she was afebrile, but exhibited a rapid heart rate at 116 beats per minute and a blood pressure of 102/78mmHg. Her respiratory rate was 20 breaths per minute and her oxygen saturation was 92% on room air. Chest auscultation revealed clear air entry in both lungs with no additional sounds. The rest of the examination was unremarkable.
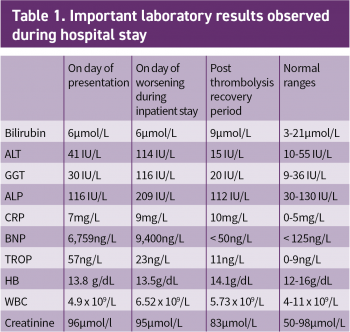
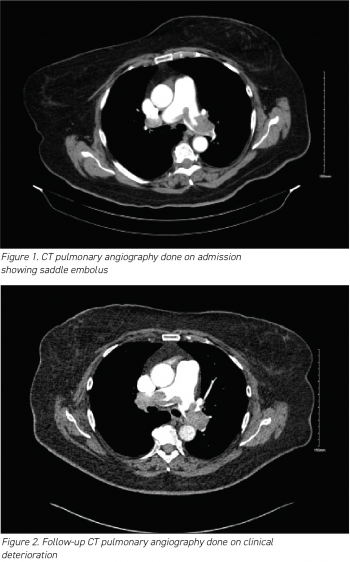
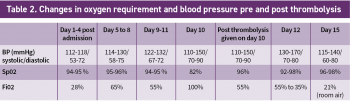
The results of her routine initial blood investigations are depicted in Table 1. Her initial blood workup revealed elevated D-dimers at > 4.00µg/ml (normal range 0.01 to 1.00µg/ml).
 (click to enlarge)
(click to enlarge)

 (click to enlarge)
(click to enlarge)
 (click to enlarge)
(click to enlarge)