CANCER
MENTAL HEALTH
Mental health in hereditary breast and ovarian cancer syndrome patients
Mental healthcare provision for patients at high genetic/familial risk of breast/ovarian cancer presents a new challenge in service delivery
November 21, 2014
-
There has been a marked increase in referral numbers of patients, who are at genetic and high familial risk of breast and ovarian cancer, from the surgical and medical oncology teams who deliver the breast cancer service to the psycho-oncology service within the Department of Psychological Medicine at St James’s Hospital, Dublin. Breast cancer is both the most common cancer and also the most common cause of cancer death in women globally.1 Indeed, the lead cause of death in women between the ages of 40-55 years is breast cancer.2
This article will explore the mental health needs in the hereditary breast and ovarian cancer population identified by research to date, in particular relating to patients who are considering risk reduction options. Also presented here are the results of an audit of referrals for outpatient assessment to the psycho-oncology service in St James’s Hospital of genetic/familial high-risk breast/ovarian cancer patients. These data include referral numbers, mental health diagnosis, mental health follow-up, as well as qualitative information on reasons for referral. Lastly, a proposed model of service delivery is outlined.
Genetic testing
For many patients with breast cancer, there is a multigenerational history of the disease within the family, adding another layer of significance to receiving news of either a diagnosis or a raised genetic risk of the disease. However, inherited genetic mutations are responsible for less than 10% of all breast cancers and less than 15% of all ovarian cancers.3 The majority of these mutations, which are inherited in an autosomal dominant pattern, are in the breast cancer type 1 (BRCA1) and type 2 (BRCA2) genes, with a minority being accounted for by other genes such as the PTEN and TP53 genes.4
The Manchester scoring system is widely used to identify those in whom genetic testing is merited.5 However, those identified as requiring genetic testing may or may not choose to proceed. Perceived risk rather than actual risk appears more important in motivating patients to choose to be tested. While pre-test worry and distress raised likelihood of requesting a test, pre-test depression reduced likelihood. Worries about negative consequences in job and insurance applications also mitigated against test uptake.6
Different countries and centres operate differing systems of genetic testing and psychological support.7 For example, Hopwood et al reported that in St Mary’s Hospital in Manchester, patients have two genetic counselling sessions if the patient is known to the service, or three if the patient is not known to the service, with a follow-up consultation to discuss surgical management.8 Areas covered in the consultations include risk evaluation, medical implications, possibility of ambiguous results, psychological risks and benefits and risk in descendants. The authors also noted that a genetic counselling service is often ‘pieced together’, and that patients reported a sense of being unsupported between appointments. A recommendation is that a dedicated advanced nurse practitioner should co-ordinate the service in order to improve continuity.8
Psychological impact of testing
It appears that at different time points, patients experience the impact of genetic testing, and the positive or negative results thereof, quite differently. Baseline psychological functioning in genetic counselling candidates compares to that of primary care populations.9
Overall, studies following patients for a year or more indicate that non-carriers experience considerable benefits from testing, and that carriers are no worse off.10 Women receiving a carrier test result experience more anxiety immediately post-test than those in receipt of a non-carrier result. However, women with a non-carrier result who have a sister who has received a carrier result can experience post-test depression.11 General and cancer-specific distress at 18-month follow-up is better predicted by pre-test distress and neuroticism than by a carrier status test result.12
Another study found that while mutation carriers endorsed more depressive symptoms, negative mood and cancer-specific distress at one and six months post-test, levels were at or approaching baseline by one year.13 Watson et al followed up carrier/non-carrier patients at one, four and 12 months post-testing, and found that while both carriers and non-carriers had returned to worry levels lower than that at baseline, non-carriers returned to a lower level than carriers.14
Interestingly, a longer-term study, following carriers and non-carriers up to five years, found that anxiety and depression increased in both groups between one and five years. However, those who had prophylactic surgery had less cancer-related worry.15 In this study, it was also found that long-term distress was associated with less intra-familial communication about testing, changes in family relationships, doubt regarding test validity and higher risk perception. A consistent finding is that pre-test distress and anxiety correlate highly with post-test symptoms.16,17
Prophylactic mastectomy, chemoprevention and surveillance
Following receipt of a mutation carrier test result, decisions need to be made about what sort of measures, if any, individuals might select to reduce cancer risk. Surveillance involves regular personal and healthcare professional breast examination, annual mammography and breast magnetic resonance imaging (MRI) from the age of 25-30.18 Surveillance reduces mortality by 46%.19 Chemoprevention consists of prescription of selective oestrogen receptor modulators (tamoxifen, raloxifene) for five years, with an expected risk reduction in post-menopausal women of 50%.20 Lastly, bilateral prophylactic mastectomy removes most breast tissue, and reduces risk of breast cancer by at least 90%.21
Each of these strategies carries advantages and disadvantages that should be communicated to the patient in terms of risk reduction, invasiveness, cost, inconvenience and whether or not it leaves open the possibility of opting for a different strategy in the future.
Patients who choose prophylactic mastectomy (PM) were found in one study to have higher distress pre- and post-test than patients opting for surveillance. However, these differences were non-significant by one year.11 The same study also found that a decision to opt for prophylactic mastectomy correlated with having young children, being in one’s 30s and longer duration of awareness of genetic risk in the family. It did appear, however, to have a negative impact on body image, intimate relationship and physical wellbeing.11
A study comparing those opting for PM versus other methods of risk reduction found that psychological morbidity decreased in the PM group, but not in the other groups. Those opting for other risk reduction methods were more likely to suffer from anxiety, but to believe in the efficacy of screening.22 Another study also found, as in the study by Lodder et al,11 a reduction in distress from pre-PM to six-month and six- to nine-year follow-up. Again, some degree of body image disturbance was found, with poor body image pre-PM predicting poor body image at follow-up.23
A study looking at those choosing PM found that 70% were satisfied with the result at long-term (mean 14.5 years), with 11% being neutral and 19% dissatisfied. At follow-up, PM was also associated with less cancer-related worry, and no impact or favourable impact on emotional stability, stress, self-esteem, sexuality and femininity.24
One study found that while anxiety reduced over the course of a year post-PM, depression did not, and also noted a disimprovement in body image and sexual functioning at one year.25 A study carried out by Hopwood et al8 found that at follow-up, post-PM women had similar General Health Questionnaire scores to women who had chosen surveillance. Half of the post-PM women had a small disimprovement in body image and approximately a third of women felt slightly less feminine.
Surgical complications were associated with poorer outcomes, and may represent a group requiring additional psychological support. Another study found that 89% of post-PM patients were satisfied with the outcome and, again, that dissatisfaction was correlated with post-surgical complications or poor cosmetic result.26
Metcalfe et al found that 97% of patients were satisfied post PM, but that younger women were less satisfied than older women.27 A postal survey with a 56% response rate of post-PM versus surveillance patients found that 84% of post PM patients were satisfied compared to 61% of surveillance patients.28
St James’s audit
Methods
An audit was carried out of outpatient referrals to the psycho-oncology service in the Department of Psychological Medicine in St James’s Hospital, Dublin, of patients who met the inclusion criteria of:
- Genetic/familial high-risk patient referred by breast cancer service in risk reduction planning phase
- Referred to psycho-oncology outpatient clinic for assessment
- Assessment appointment offered between January 2010 and March 2014.
Sources of information used to extract the relevant data included the online first assessment clinic list, the detailed first assessment letter and clinical case notes. More detailed qualitative information about referral triggers was gleaned from interview with breast cancer service medical and surgical oncology consultant colleagues.
Results
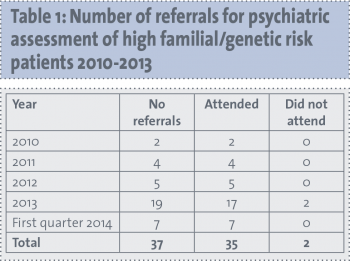
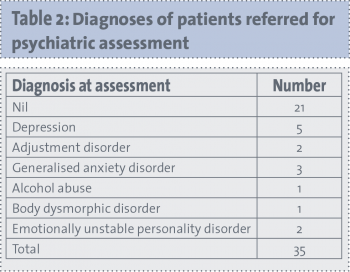
In 2010 there were two referrals of high familial/genetic risk patients in the planning phase of risk reduction treatment, increasing over the intervening year (see Table 1) to 19 referrals in 2013. In the first quarter of 2014, there were seven referrals. Data refer to the 35 patients who attended the clinic between January 2010 and March 2014. Discussions with consultant colleagues indicated that their referral triggers included the following: concerns about patient distress, history of mental illness, poor social support, difficulty with decision-making regarding treatment, and baseline assessment in those in whom there was concern there could be surgical complications. The mean age of patients being referred was 39.4 years, of whom 74.3% had children. Patients were assessed by a non-consultant hospital doctor/clinical nurse specialist and the psycho-oncology consultant psychiatrist.
 (click to enlarge)
(click to enlarge)

 (click to enlarge)
(click to enlarge)
 (click to enlarge)
(click to enlarge)