CANCER
Non-small cell lung cancer
ROS1 rearrangement is present in a small subset (1-2%) of NSCLC and is associated with slight, never-smoking patients and adenocarcinoma histology
May 1, 2018
-
Although chemotherapy drugs remain the backbone of current treatment strategies they are limited by a narrow therapeutic index, between lack of efficacy and dose-limiting toxicity as well as both innate and acquired resistance. An improved understanding of the underlying molecular drivers of lung cancer pathogenesis has led to the identification of new mechanism-based treatment options. Currently, there are several novel therapies in clinical practice, including those targeting actionable mutations and more recently immunotherapeutic agents.
Targeted therapies such as EGFR, ALK and ROS1 tyrosine kinase inhibitors (TKIs) switch off specific aberrant signalling pathways that are crucial for tumour growth and maintenance; whereas immunotherapy harnesses the endogenous immune system to mount an anti-tumour response. Recent clinical trials of immune-checkpoint inhibitors (ICPIs) targeting CTLA-4, PD-L1 or PD-1 have shown remarkable clinical efficacy in the treatment of metastatic lung cancer. However, only approximately 30% of patients respond to immunotherapies. Initial evidence with combined inhibition of PD-1 and CTLA-4 has highlighted the potential of combining agents with synergistic mechanisms of action. Targeted therapies and cytotoxic agents also modulate immune responses, which raises the possibility that these treatments might be effectively combined with immunotherapy to improve clinical outcomes for patients with lung cancer.
Targeted therapies
The discovery of targetable gene alterations has dramatically changed the treatment of lung cancer.1,2 Molecular profiling of tumour tissue is now incorporated into patient diagnosis to allow for more tailored therapy. Mutation profiling of circulating tumour DNA (ctDNA) and circulating tumour cells (CTCs) are becoming increasingly promising options for minimally invasive cancer therapy prediction and disease monitoring. This individualised approach to treatment has led to remarkable responses in selected patients treated with matched TKIs. Results from the Lung Cancer Mutation Consortium highlighted targetable oncogenic drivers in 64% of patients with adenocarcinoma and the use of mutation-directed therapy was associated with improved survival compared to those treated without targeted therapies.3
Epidermal growth factor receptor (EGFR)
EGFR is a member of the receptor tyrosine kinase family that includes human epidermal growth factor receptor 2 (HER2, also known as ERBB2), HER3 (ERBB3) and HER4 (ERBB4). Somatic mutations in the EGFR TK domain proved to be a predictor of response to EGFR TKIs and were first described in 2004. First-generation reversible EGFR TKIs, including gefitinib and erlotinib, showed higher objective response rates (ORRs) and progression-free survival (PFS) compared to cytotoxic therapy in previously untreated patients with EGFR mutations.4 Second-generation irreversible inhibitors that also target HER2 and HER4 such as afatinib and dacomitinib resulted in improved PFS compared to gefitinib.5,6 The EGFR (T790M) mutation is the most common cause of acquired resistance to first-generation TKIs. Other mechanisms of resistance include HER2 amplifications, mutations in MET, BRAF or phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), and SCLC transformation. Molecular profiling at progression is required to identify follow-up treatment.7
Third-generation EGFR TKIs are selective inhibitors of both the original sensitising and T790M mutations, while sparing wild-type EGFR. Osimertinib, a third-generation EGFR TKI, is effective in patients harbouring the EGFR (T790M) mutation following progression after first generation EGFR TKI, and showed increased ORRs and PFS compared to platinum-based chemotherapy.8
In a randomised trial comparing osimertinib to either erlotinib or gefitinib in previously untreated patients with advanced stage NSCLC harbouring either EGFR exon 19 deletion or L858R mutation, osimertinib was associated with a significant improvement in PFS, establishing it as a first-line EGFR TKI option. The C797S mutation is a mechanism for acquired resistance to third-generation EGFR TKIs but not to gefitinib or afatinib.
Fourth-generation allosteric inhibitor EAI045 provides a promising approach to triple mutants (sensitising mutation, T790M and C797S). Brigatinib is an ALK inhibitor that also has activity against triple mutant EGFR. The efficacy of brigatinib and EAI045 are enhanced markedly by combination with anti-EGFR antibody cetuximab because of the decrease of surface and total EGFR expression.9,10
Anaplastic lymphoma kinase (ALK)
ALK gene rearrangements occur in about 4-5% of all NSCLC patients. The most common fusion partner is the echinoderm microtubule-associated protein-like 4 (EML4) gene (EML4-ALK). Crizotinib – an oral competitive ATP inhibitor of ALK, MET and ROS1 tyrosine kinases – is associated with improved ORRs and median PFS compared to chemotherapy in both previously treated and untreated patients. Most patients previously treated with crizotinib benefit from second-generation ALK inhibitors including ceritinib, alectinib and brigatinib. Ceritinib also increased median PFS compared to first-line chemotherapy in ALK-positive patients.
Two randomised studies showed increased ORRs and median PFS for alectinib compared to crizotinib in patients with previously untreated ALK-positive NSCLC, establishing alectinib as a first-line treatment option.11,12 Resistance to ALK inhibitors may occur due to alterations in ALK such as mutations and amplification, or upregulation of bypass signalling pathways including EGFR and mitogen-activated protein kinase (MAPK).
The G1202R mutation is the most common ALK resistance mutation among patients treated with second-generation TKIs and results in in vitro resistance to all currently available ALK inhibitors except for lorlatinib, a potent third-generation ALK inhibitor.13
ROS proto-oncogene receptor 1 (ROS1)
ROS1 rearrangement is present in a small subset (1-2%) of NSCLC and is associated with slight, never-smoking patients and adenocarcinoma histology. The ROS1 gene encodes a receptor tyrosine kinase that is constitutively activated when a rearrangement leads to the fusion of its tyrosine kinase domain with a partner gene such as CD74, EZR, FIG1, CCD6, KDELR2, LRI3, SDC4, SLC34A2, TPM3 and TPD52L1.
ROS1 is closely related to LTK and ALK. Drugs used to treat ALK-positive tumours including crizotinib, ceritinib and lorlatinib have also shown marked activity in ROS1-positive tumours. Mechanisms of acquired resistance of ROS1 rearrangements to crizotinib include secondary mutations, most commonly G2032R, wild-type EGFR signalling activation, KRAS and KIT mutations.14
Other targets
MET exon 14 alterations resulting in increased MET protein levels due to disrupted ubiquitin-mediated degradation, occur at a prevalence of around 3% in adenocarcinomas and around 2% in other lung neoplasms. MET exon 14 splicing abnormalities, causing the loss of the MET juxtamembrane (JM) domain, recently emerged as a new potential oncogenic driver. Patients harbouring MET exon 14 skipping may respond to MET inhibitors including crizotinib, cabozantinib, capmatinib, tepotinib and glesatinib.15,16
Mutations in BRAF are observed in 2-4% of NSCLCs and typically lead to constitutive activation of the protein and the mitogen-activated protein kinase (MAPK) signalling pathway. BRAF-mutant NSCLC frequently harbours the V600E allele (~55%). Other highly frequent activating BRAF variants include G469A (~35%) and D594G (~10%). The V600E mutation predicts sensitivity to the BRAF inhibitors vemurafenib and dabrafenib as single agents, or dabrafenib in combination with the MEK inhibitor trametinib.17
Other potential targetable gene alterations include mutations in HER2, rearrangements in the proto-oncogene RET, which encodes a receptor tyrosine kinase, and fusions of the neurotrophic tyrosine receptor kinase (NTRK) genes 1, 2 and 3, which code for tropomyosin receptor kinases (TRK) A, B and C, respectively. Targeted therapies against HER2 and RET alterations have shown modest activity compared to other oncogenic targets highlighting their roles as dominant clonal drivers.18,19 TRK TKIs have demonstrated histology-agnostic efficacy in patients with NTRK fusion-positive cancers, which occur in less than 1% of NSCLC.20
Immunotherapies
Harnessing the immune system and its components to mount an anti-tumour response is not a new concept, however modern immunotherapy approaches focus on bolstering T cell responses and augmenting cell-mediated immunity to potentiate tumour destruction. This new approach known as immune checkpoint blockade (ICB) began with the development of monoclonal antibodies that target cytotoxic T-lymphocyte antigen-4 (CTLA-4) (ipilimumab and tremelimumab) and was rapidly followed by antibodies against the programmed death-1 (PD-1) T cell co-receptor (nivolumab and pembrolizumab) and its ligand, B7-H1/PD-L1 (durvalumab, atezolizumab, avelumab and others).21
ICB has shown significant benefit in a broad population of patients with NSCLC and has been approved as a standard of care for patients with advanced NSCLC whose tumours progress on first-line chemotherapy. Treatment with nivolumab was associated with significantly longer median overall survival compared to treatment with docetaxel among patients with metastatic NSCLC who had disease progression during or after platinum-based chemotherapy.22
In the first-line setting, pembrolizumab was established as a new standard of care for patients with advanced or metastatic NSCLC with tumour PD-L1 expression levels of 50% or more (by a companion immunohistochemistry test), which is present in up to 30% of NSCLC.23 In these patients, pembrolizumab is associated with a significant improvement in ORRs, PFS and overall survival compared to platinum-based chemotherapy. By contrast, among patients with PD-L1 expression levels of 5% or more, nivolumab was not associated with improvements in PFS or overall survival compared to chemotherapy.24
Among patients in the nivolumab group with both PD-L1 expression levels above 50% and high tumour mutation burden (TMB), the ORR was 75%, confirming previous observations that both TMB and PD-L1 expression may predict for benefit from ICPIs. Better toxicity profiles with ICPIs and equivalent survival outcome could support their selection as first-line therapy for patients with PD-L1-expressing advanced NSCLC, especially in those not eligible for chemotherapy.25 The notable clinical success of cancer immunotherapy over a short period of time suggests that it may form the foundation of future curative intent regimens for NSCLC.
Combination strategies
Despite encouraging results with prolonged benefit in selected patients, most lung tumours are either inherently resistant or will adapt to or become resistant to current immunotherapies. The challenge for clinicians is to develop rational combinations that will increase responses, or delay the onset of resistance. Combining anti-CTLA-4 and anti-PD-(L)1 monoclonal antibodies may result in more durable responses in NSCLC, as suggested in a single-arm clinical study.26 Randomised studies that combine durvalumab with the anti-CTLA-4 human IgG4 antibody tremelimumab and, nivolumab with ipilimumab, are continuing and overall survival data from these studies are eagerly awaited.
It is expected that the safety profile for such combinations may not be as favourable as those with ICPI plus chemotherapy combinations, especially in terms of immune-related adverse events. Although ICPI monotherapy may be the best approach for treating tumours with high PD-L1 expression or with a high nonsynonymous TMB, a different approach may be required for tumours with fewer T cells and a lower TMB.
Combining targeted therapy with immunotherapy
Targeted therapies have demonstrated dramatic but transient responses in a subgroup of patients and immunotherapy can provide durable responses in a subset of patients however both treatments options are limited by the inevitable emergence of resistance. As well as the direct killing of tumour cells, targeted therapy also has effects on different components of the immune system, so called immune sensitisation. This ability to ‘prime’ the immune system suggests a potentially synergistic benefit of combining targeted therapy and immunotherapy beyond the expected additive effect of two effective treatments.
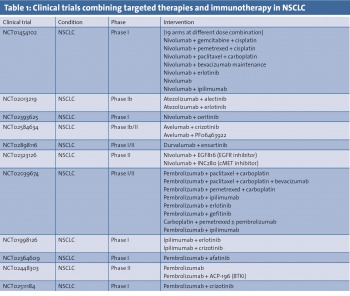
For example, the EGFR mutation has been shown to mediate immune escape through the upregulation of PD-1 and PD-L1 in lung cancer27 and treatment with EGFR inhibitors reduced PD-1 and PD-L1 expression.28 These data have been instrumental in the establishment of several clinical trials focusing on the combination of EGFR and PD-1 inhibitors (highlighted in Table 1). However, it is too early to predict the outcome of this strategy and we are mindful that this constitutive expression of PD-L1 is a result of an oncogenic event as opposed to adaptive immune expression. Therefore, while blocking this driver mutation might downregulate the expression of PD-L1 in the tumour, it remains unclear what the consequences are in tumours that lack infiltrating lymphocytes, which is more common in EGFR mutant NSCLC.29
 (click to enlarge)
(click to enlarge)

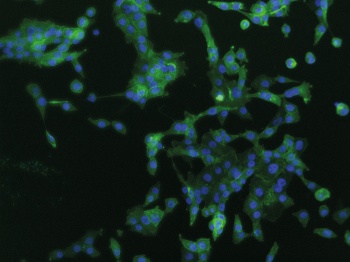 PD-L1 expression in EGFR mutation positive H1975 cells (click to enlarge)
PD-L1 expression in EGFR mutation positive H1975 cells (click to enlarge)
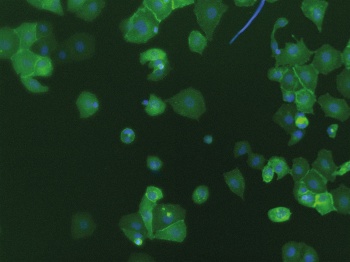 PD-L1 expression in TKI resistant H1975 cells (click to enlarge)
PD-L1 expression in TKI resistant H1975 cells (click to enlarge)