CANCER
Novel agents represent paradigm shift in chronic lymphocytic leukaemia treatment
Chronic lymphocytic leukaemia is the most common leukaemia seen in the western hemisphere and approximately 200 to 300 new cases are diagnosed across Ireland each year
December 1, 2015
-
Chronic Lymphocytic Leukaemia (CLL) is the most common leukaemia seen in the western hemisphere. It accounts for 38% of all leukaemia types combined and has an annual incidence of about four in every 100,000 people, therefore approximately 200 to 300 new cases are diagnosed across the island of Ireland every year. It is generally a disease of older persons; about 40% of new cases are diagnosed in people over the age of 75, with a male preponderance. Fortunately, around one-quarter of affected individuals will only ever require observation and appropriate vaccinations for influenza and pneumonia as their disease will not progress sufficiently to cause them harm and thereby necessitate anti-cancer treatment.1
The remaining 75% of individuals will require, at some stage of their disease, a therapeutic intervention and the choice of therapy may be limited by their frequently-seen advanced years and comorbidities.2,3
In addition, response to treatment in CLL patients is extremely heterogeneous, with some patients having long and durable remissions, in some cases for more than 10 years, but others less fortunate will have short remissions or worse still not respond to therapy at all.
Cytogenetic abnormalities
This variable natural history and response to therapy in CLL is partly predicted by clinical and genomic features; these prognostic markers increase with each year and each new international conference. To avoid an exhaustive list, the three main chromosomal aberrations worth noting are: deletion of the long arm of chromosome 13, 13q14 deletion; deletion of the long arm of chromosome 11, 11q23 deletion; and deletion of short arm of chromosome 17, 17p deletion.
Contained on the short arm of chromosome 17 are the genetic instructions for the manufacture of the TP53 protein, essential for promotion of apoptosis (cell death) in cells damaged by chemotherapy or radiation. Not surprisingly, all cancer patients, not just CLL patients, with absence or abnormalities of this protein have a much worse outcome. In CLL, median survival for treated patients with standard chemotherapeutic approaches has been as little as 18 months.
The 11q23 deleted patients fare only slightly better and patients with 11q23 or 17p deletions are considered to have a ‘poor risk’ cytogenetic profile, whereas those with 13q14 do extremely well if this is their sole abnormality; they frequently never require treatment, can have normal life expectancy, and are appropriately described as ‘good risk’.4
Evolving CLL therapies
Therapy for CLL has evolved from monotherapy with alkylating agents, such as chlorambucil, to purine nucleoside analogues such as fludarabine.
Fludarabine as a single agent was shown to be superior to chlorambucil and its incorporation into combination regimens such as the FC (fludarabine and cyclophosphamide) reported 80% response rates. Addition of anti CD 20 antibody rituximab improved treatment further still and the fludarabine cyclophosphamide rituximab (FCR) regimen became the new gold standard CLL treatment.5
However, this treatment was not without its toxicity and many older individuals where unfit for this regimen. Also, responses in ‘poor risk’ patients were short or unsatisfactory.
As a consequence, until recently, the two greatest challenges in the management of this leukaemia have been:
• The unsatisfactory responses and remission duration in poor risk CLL patients
• The age and existing comorbidities of the patient requiring therapy.
Advances in anti-cancer treatment have come a long way in the past few years towards addressing these problems with impressive success. Four new agents have come to the fore in the treatment of CLL that have changed the treatment paradigm entirely from chemotherapy to a more targeted approach, which has reduced toxicity, enabling older and less fit patients to avail of treatment. Efficacy has also improved, in particular, overcoming the previous hurdles of poor risk such as the negative impact of 17p deletion and p53 dysfunction. The novel treatments are obinutuzumab, ibrutinib, idelalisib and ABT-199/GDC-0199.
These agents have left behind the traditional methodology of cell killing by cytotoxicity requiring intact p53 apoptotic pathway, and are effective by a more targeted approach. The drugs work on the knowledge and manipulation of the following molecular biology:
• CLL is a malignancy of B cells, which express the CD20 antigen
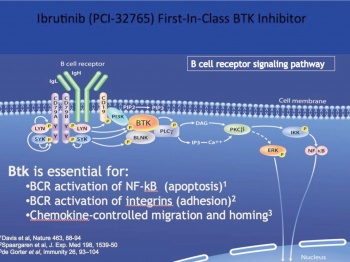
• CLL cells, similar to normal non-malignant B cells, are dependent on their continued survival, migration and proliferation, on stimulation of the B cell receptor (BCR) and up regulation of NF Kappa B pathways via tyrosine kinases such as Bruton’s tyrosine kinase and PI3 kinases6
• CLL cells like many lymphoid malignancies are protected from apoptosis by the anti cell death regulatory protein BCL-2 (B cell lymphoma 2). This is over expressed in many lymphoproliferative disorders including CLL, and BCL2 is inhibited by the protein BH3 only protein.7
Obinutuzumab (Gazyvaro); Roche
Obinutuzumab is a novel, fully humanised, glycoengineered type II monoclonal antibody that targets the CD20 receptor on mature B cells.
In a phase III clinical trial, which included 671 patients with CLL and significant comorbidities, patients treated with obinutuzumab–chlorambucil combination had longer median progression-free survival than those treated with chlorambucil alone (26.7 months versus 11.1 months, p < 0.001) or rituximab plus chlorambucil (26.7 months versus 15.2 months, p <0.001). A limited course of six cycles of obinutuzumab plus chlorambucil showed superiority over rituximab plus chlorambucil in terms of complete response (20.7 versus 7.0%), and rate of negativity for minimal residual disease, both in peripheral blood (37.7 versus 3.3%) and bone marrow (19.5 versus 2.6%). The main adverse effect associated with obinutuzumab was infusion-related reactions (IRRs), occurring in 66% of patients in the phase III trial. Grade 3 or 4 IRR occurred in 20% of patients during the first infusion of obinutuzumab, but there were no grade 3 or 4 reactions during subsequent obinutuzumab infusions; IRR risk can be reduced by appropriate pre-medication, dosing and patient monitoring.8
Ibrutinib (Imbruvica); Janssen
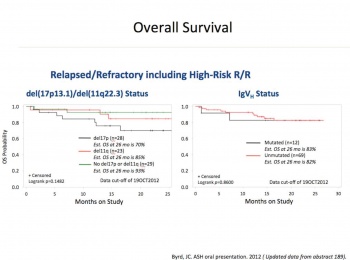
Ibrutinib is an oral covalent inhibitor of Bruton’s tyrosine kinase (BTK). BTK is a kinase active by BCR stimulation and necessary for proliferation survival and migration of CLL cells. Phase I and II studies in the US demonstrated that ibrutinib was an effective treatment for CLL (see Figure 1), even in chemo refractory cases and those with p53 deletions. Better still, as this is a small molecule it can be give in tablet form, and as it was not a cytotoxic agent, the usual side effects of severe myelosuppression and alopecia were not present (Figure 2).
CLL patients in Ireland were among the 391 patients who participated in Resonate, a multinational phase III study, which launched in June 2012. Indeed, the very first person to receive ibrutinib for CLL in Europe was an Irish woman and a patient in Beaumont Hospital, Dublin. This study, which was recently published in the New England Journal of Medicine, compared ibrutinib and ofatumumab, an anti CD20 monoclonal antibody. The regulatory body closed the study early as ibrutinib was found to be clearly superior with improved progression-free and overall survival of 90% versus 81% at 12 months (p = 0.005). Similar responses were seen regardless of whether patients had a 17p deletion or were refractory to purine analogues.
Adverse events of grade 3 or higher that occurred more frequently in the ibrutinib group were diarrhoea (4%) and atrial fibrillation (3%). Bleeding-related events, most commonly petechiae or bruising, were more common with ibrutinib (44%). Major haemorrhage of grade 3 or higher occurred in only two patients (1%) versus 2% in the ofatumumab group.9
Ofatumumab patients were allowed to cross over and receive ibrutinib, and an expanded access programme was available in Ireland for refractory and p53 deleted patients until March 2015.
 (click to enlarge)
(click to enlarge)

 (click to enlarge)
(click to enlarge)