GENERAL MEDICINE
PET – latest techniques in nuclear imaging
PET has fundamentally changed not only the assessment and treatment of patients but how we conceptualise disease itself
January 1, 2011
-
Though it was born in the 1970s most of us did not see positron emission tomography (PET) in clinical practice until the late 1990s. Given the very complex and expensive technology involved it was initially confined to those units that were heavily involved in its development.
My first encounter with PET was in such a unit at the Mallinckrodt Institute of Technology, St Louis, Missouri, in the US. But rather like the iPhone and iPad, it arrived with a combination of high impact and utility such that quickly few of us could understand how we worked without it.
The major role of PET in the diagnostic evaluation process was universally acknowledged and its momentum gathered pace so that combined PET-CT (PET-computed tomography) today is very much a mainstream tool in radiology practice. Manufacturers have helped ensure the technology is now accessible to average-sized hospitals and imaging centres.
This technology has fundamentally changed not only how we assess patients and treat them but how we conceptualise the disease itself. It has shifted modern imaging development of superlative anatomic detail into the realm of metabolic and functional assessment of the body. In this paper we shall discuss a brief overview of PET-CT practice and then discuss its major applications in oncological and non-oncological diseases processes in 2011.
What is PET-CT?
PET is a particular nuclear medicine imaging tool that utilises positron (an electron-like particle but with a positive charge) emitting radionuclide agents. At their site of uptake they emit two photon rays (called gamma rays) 180 degrees opposite to one another. This permits an image reconstruction reflecting the distribution of activity even though the sites of uptake are in very small picomolar quantities.
Such PET radionuclides include carbon, nitrogen, oxygen, fluorine, galium and rubidium which can be used to label most biomolecules without changing their constitution or properties.
F-fluorodeoxyglucose (FDG) is the only agent used commonly in clinical practice as it is easily produced and its relatively long half-life permits ease of distribution. FDG has been proven to be of high utility and broad application and is now firmly part of medical vernacular among all staff grades.
This agent is administered intravenously and taken up and trapped within tissues according to their glucose utilisation. Diseased tissues tend to have a high rate of glucose metabolism compared to background tissues.
At a cellular level many enzymatic changes have been documented including increased glucose transport rates and phosphorylation and decreased de-phosphorylation.
We can get an indirect measure of FDG uptake (standardised uptake value; SUV) which can be compared between scans on individual patients and between patients. This quantification is becoming increasingly important in tissue differentiation and assessing tissue biology and response.
Modern multi-slice CT
Though PET machines (see Figure 2) were originally stand-alone, all modern PET platforms combine with computed tomography (CT) scanners (PET-CT). Such hybrid imaging was of course a reinvention of two wheels capitalising on the synergy of each modalities’ respective strengths. Modern multi-slice CT technology provides superb high resolution anatomic detail in three dimensions as well as providing a means to improve the PET image quality (attenuation correction).
PET-CT is easily performed as an outpatient study. Quality imaging does require a specialist team of radiologist, radiographer, nursing and support staff. There is a specific patient preparation process and patients may receive conventional iodinated contrast as well as FDG.
The protocols between patients are broadly similar but there may be some variation to reflect the disease process under study. Behind the scenes there is a large amount of work involved in regulatory compliance and safety given the use of radiation and radionuclides on patients.
Ireland is fortunate to already have a number of PET-CT centres with superbly trained individuals providing high quality care with this latest technology equivalent to anywhere in the world.
Role of PET-CT
The major role of PET-CT internationally in current radiology practice is in cancer care. It also has an emerging role in neurological, cardiac and inflammatory diseases. In specialised PET-CT centres its role may extend into other limited areas and disease states, but the more common and widely accepted roles are discussed here.
Oncology
The tools (eg. radiography, ultrasound; US, CT, magnetic resonance imaging; MRI, bone scan) available in most imaging departments in Ireland now suffice to provide a very comprehensive, detailed and accurate assessment of most cancers presenting in hospital practice.
Indeed not all cancers need PET-CT but rather PET-CT is applied when one thinks its unique means of characterising a disease state will effect a management change through tissue sampling, treatment or prognosis.
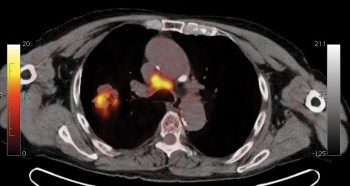
Fortunately PET-CT seems to work best in those cancers commonly seen in our society including: lung (see Figure 4), breast, lymphoma (see Figure 3), gastrointestinal (GI; colorectal, oesophageal; see Figure 1), head and neck, gynaecological (uterus, cervix, ovary), melanoma as well as some less common tumours.
Though there are broad agreements there are as yet no universally accepted algorithms for the use of PET-CT. For guidance, practitioners look to evidence-based studies, consensus statements from professional bodies, specialty journal recommendations and US insurer/medicare policy. The who, when and why questions are always difficult when such population guidance is filtered down to individual patients.
It has a role in lesion characterisation, diagnosis, tissue sampling, staging and assessment of treatment response.
PET-CT is not commonly used to diagnose cancer, in fact the majority of patients that present for scanning already have a suggested diagnosis. In such cases it mainly serves to further characterise the primary disease through its activity, location and pattern etc.
In select conditions PET-CT can help differentiate benign and malignant lesions eg. a solitary lung nodule and non-small cell lung cancer (NSCLC). PET-CT has been used to search for primary lesions in patients traditionally diagnosed as ‘adenocarcinoma of unknown primary’.
In exceptional circumstances PET-CT has been employed to search for a primary oncological process in a patient with a highly suggestive clinical syndrome where traditional methods have failed to yield a causative lesion. Finally, as with the advent of all new imaging technologies PET-CT has brought with it, its own so-called ‘incidentalomas’. This is where the PET-CT is done to evaluate a specific known cancer and yet incidentally picks up a separate synchronous primary lesion.
In some lesions when tissue diagnosis is required the PET-CT can be of value. The areas of greatest FDG avidity can be used to guide the operator to the site most likely to yield active tumour cells decreasing sampling error. Examples would include sampling of large tumours with extensive areas of necrosis where the lesion is outgrowing its blood supply. Another is in large infiltrating lung lesion centred in collapsed lung where the activity can help delineate the site of lesion versus that of adjacent collapsed lung.
Finally, depending on the individual case, local practice and management algorithms multidisciplinary teams will on occasion accept PET-CT features as pathognomonic for primary and metastatic disease and obviate the need for tissue sampling altogether.
For example some centres will operate on an isolated lung lesion which has morphologic and PET features of bronchogenic carcinoma in the absence of tissue given the test’s high positive predictive value.
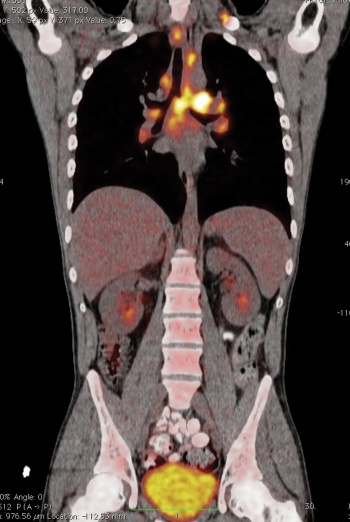 Figure 3. Body scan showing lymphoma(click to enlarge)
Figure 3. Body scan showing lymphoma(click to enlarge)

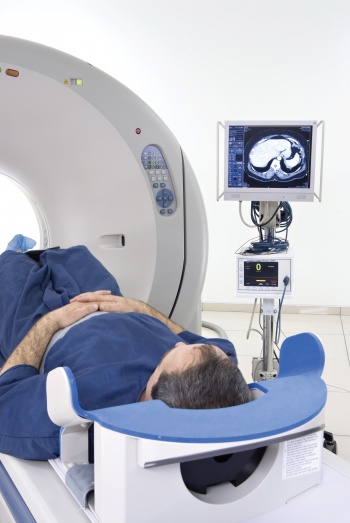 Figure 2. Patient undergoing PET scan(click to enlarge)
Figure 2. Patient undergoing PET scan(click to enlarge)
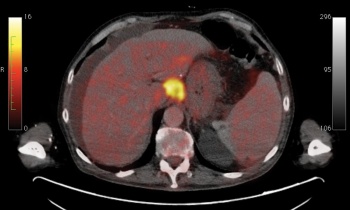 Figure 1. Oesophageal carcinoma.(click to enlarge)
Figure 1. Oesophageal carcinoma.(click to enlarge)