OBSTETRICS/GYNAECOLOGY
WOMEN’S HEALTH
Predicting pre-eclampsia in first-time pregnancies
Cork researchers are at the forefront of an international study aimed at the early detection of pre-eclampsia
January 7, 2014
-
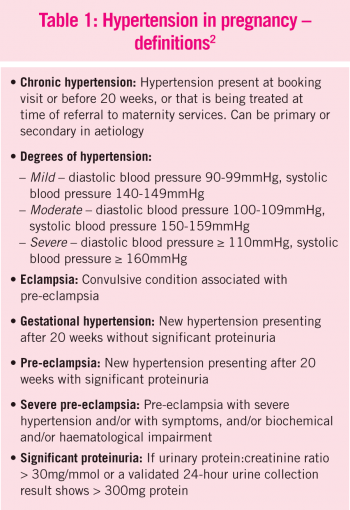
Pre-eclampsia is the leading cause of maternal death in Europe and the cause of over 500,000 infant deaths annually worldwide.1 With pre-eclampsia, blood pressure can reach dangerously high levels and protein is present in the urine. If left untreated, this can develop into eclampsia, or life-threatening seizures during pregnancy.
The condition tends to occur during the second half of a pregnancy and it usually resolves once the baby is delivered. However, more serious problems arise if the condition develops earlier in the pregnancy, as decisions may have to be made on whether to deliver the baby prematurely.
While women who have previously had the condition are at increased risk in subsequent pregnancies, predicting risk among first-time mothers is difficult.
However, a new research initiative IMPROvED (IMproved PRegnancy Outcomes by Early Detection) is aiming to develop a simple blood test for the condition.
The main goal of the IMPROvED project is to develop a clinically robust predictive blood test for pre-eclampsia, using novel metabolite and protein biomarkers. This blood test will be targeted at all first time mothers during early pregnancy to determine their risk for this major pregnancy complication.
This is thought to be the first trial internationally to test these two new prototypes for early pregnancy screening.
The blood test is carried out at 15 weeks gestation and indications are that it may be able to pick up 95% of women who will go on to develop pre-eclampsia. The trial will test this.
This project aims to impact radically on the provision of antenatal care, both in Europe and the rest of the world, and hopes to result in a reduction of clinical complications.
Consultant obstetrician and gynaecologist Prof Louise Kenny of Cork University Maternity Hospital is leading the international group of doctors and scientists. “Our goal is to save the lives of affected mums and babies by reducing and eventually preventing the life-threatening complications associated with pre-eclampsia,” she said.
“Mums-to-be can be confident that they are in excellent hands as all participating obstetric recruitment centres have well-established track records and outstanding reputations for the research and management of pre-eclampsia.”
Prof Kenny recently won the Enterprise Ireland Life Sciences and Food Commercialisation Award for developing and commercialising this first predictive diagnosis for pre-eclampsia in early pregnancy. She received the award at the Enterprise Ireland Big Ideas Technology Showcase which took place in Dublin recently.
The clinical study
A total of 5,000 first-time mothers will be recruited over the course of two years to academic medical centres across Europe (Ireland [Cork], the UK, the Netherlands, Sweden and Germany) into a phase IIa prognostic multicentre hospital-based clinical study.
Seven recruitment sites (all with high patient-throughput) have been selected on the basis of investigator expertise and background in pre-eclampsia research. The different centres will facilitate assessment of the screening test in different models of healthcare delivery.
Detailed clinical data will be collected, and blood samples taken in the first trimester, at 11, 15, 20 and 34 weeks’ gestation. Women will then be followed throughout their pregnancies and clinical outcomes will be recorded. Those being enrolled must be less than 16 weeks pregnant and cannot have high blood pressure, kidney disease or diabetes.3
Biobank
Establishing a high calibre biobank, augmented by accurate clinical metadata is also an aim of the programme, which it hopes will enable the development of predictive screening tests for pre-eclampsia and will also provide a vital resource for pregnancy researchers across Europe.
In addition, according to its researchers, MedSciNet will deliver a sophisticated web-based informatics platform, already widely used internationally for data management in clinical trials and cohort studies, to create biobank management software augmented with clinical metadata.3
The IMPROvED project is in the process of training research midwives, lab technicians, clinical academics, PhD students and postdoctoral fellows who will be involved in the study. For more information see www.fp7-improved.eu or email: improved@ucc.ie
 (click to enlarge)
(click to enlarge)

 (click to enlarge)
(click to enlarge)