CANCER
PAIN
The role of interventional therapies in cancer pain management
For opioid resistant pain there are other interventional therapies that may be suitable
November 8, 2013
-
As a result of advances in the investigation and management of cancer, survival rates are much improved in this patient population. Consequently, a large number of patients are now reporting reduced quality of life, often relating to cancer pain. Therefore, it is becoming increasing important that all physicians caring for patients diagnosed with cancer, whether in the community or hospital-based, should be aware of different and evolving treatment modalities in cancer-related pain.
Mechanisms of cancer pain
Our understanding of the mechanisms of cancer-related pain is predominantly derived from animal models. Historically, animal models have proven beneficial in understanding the mechanisms underlying other pain modalities, such as neuropathic and chronic pain. Cancer pain can be considered to be multifactorial in origin, meaning it is without a single somatic, sympathetic or neuropathic pain state. It may be understood as having an inflammatory, ischaemic and neuropathic component.
Inflammatory pain may be due to the release of a number of growth factors, cytokines, interleukins and endothelins, which directly activate primary afferent fibres.1,2 Neuropathic pain can be attributed to different aetiologies, such as infiltration of the nerves by tumour mass and treatment-related, (eg. surgery, chemotherapy). This helps explains why certain medications for neuropathic pain, such as gabapentin and amitriptyline, show some efficacy in the management of cancer pain.
The pathophysiology of cancer pain has features in common with other pain states, however, there are some unique differences. In animal models with bone cancer pain, there are reported alterations on the amount of nociceptive-specific cells and wide dynamic range (WDR) neurons, those that respond to both innocuous and noxious stimuli. Models showed an increased proportion of WDR neurons that displayed hyperexcitability to thermal and mechanical stimuli.3 These similarities suggest that while some treatment modalities in other pain states may be beneficial, there is an even wider range of treatment options that may be available to patients with cancer-related pain.
Cancer pain management
Treatment of cancer-related pain is approached using a bio-psycho-social model. This may involve patient education, altering expectations, modifying activities, community supports and cognitive therapy aimed at improving mood and sleep patterns. Historically, the primary way of managing cancer pain was treatment of the underlying pathological process, eg. bone pain secondary to metastatic prostate cancer may be improved by anti-androgen treatment; chemotherapy may reduce the mechanical/ischaemic pain associate with tumour burden; and radiotherapy has a proven role in palliation for bone metastasis.4
The WHO analgesic ladder was originally published as a method for relief of cancer pain.5 The ladder is essentially a set of guiding principles that involve a stepwise approach to the use of analgesic drugs. Step one involves non-opioid based regimes (eg. paracetamol, NSAIDs); step two involves a weak opioid (eg. codeine); and step three involves a strong opioid (eg. morphine). If a patient were to present with moderate to severe pain, it is appropriate for them to commence on strong opioids immediately rather than progress through the earlier steps. The original monograph would emphasise the importance of using strong oral opioids in the management of moderate to severe cancer pain.
The underlying principles of the WHO method to relieve cancer pain – ‘by the clock’, ‘by the mouth’, ‘by the ladder’, and ‘for the individual’ – are of paramount importance. It is also vital to note that, with the advent of other treatment modalities, each stage in the ladder may be supplemented by other interventions, such as disease-modifying medications and nerve blocks, often considered the fourth step of the ladder.
The WHO ladder is still considered the gold standard in cancer pain management. Nevertheless, since its introduction, other opioid-based medications and different formulations have emerged that also have a role to play, such as fentanyl, buprenorphine, hydromorphine and oxycodone.
However, recent guidelines published by the European Association of Palliative Care cautioned that, due to a low level of evidence, no one opioid could demonstrate superiority over another in the treatment of cancer-related pain.6 Despite this, morphine still remains the most commonly used opioid (both oral and parenteral) for the management of moderate to severe pain.7
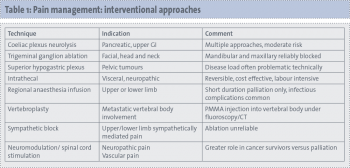
Interventional therapies
Although systemic pharmacotherapy is the mainstay of treatment for cancer-related pain, there are many limitations to this strategy; not only the opioid-based side-effects (constipation, nausea and vomiting, cognitive impairment), but more specifically an inability to provide adequate pain relief for what can be an advancing and worsening pain state (opioid unresponsive pain). For these reasons, interventional pain techniques can play an essential role in cancer pain management.8
Interventional procedures can be classified under three headings: peripheral, sympathetic and central neuroaxial techniques. They may also be re-classified as either being neuromodulatory, such as the intrathecal administration of local anaesthetic, or neurodestructive, such as the use of toxic agents, cold/heat energy or radiofrequency ablation aiming to obliterate neural function.
 (click to enlarge)
(click to enlarge)
