CANCER
Therapeutic advances in immune checkpoint inhibitors
Refining treatment combinations and identifying effective biomarkers to enhance clinical efficacy in immunotherapy remains a priority
November 1, 2016
-
Huge optimism has been stirred in the medical oncology community in recent years with breakthroughs in immunotherapy leading to durable disease response across certain cancer types. Continuing to refine treatment combinations and identify effective biomarkers to enhance clinical efficacy in this area remains a priority.
An effective cytotoxic immune response against a cancer cell requires a complex interaction between the adaptive and innate immune system. The human immune system is an intricate and sophisticated system that is not completely understood. CD8+ and CD4+ lymphocytes initiate the distinction between self and non-self antigens. Tumours evade immune surveillance through multiple mechanisms including:
• Loss or alteration of antigenic machinery1,2
• Promotion of an immune-tolerant micro-environment by manipulation of cytokines
• Upregulation of immune checkpoint molecules such as PD-1 and PD ligand 1 (PD-L1) that promote peripheral T-cell exhaustion.3
Ongoing study is directed at using the immune system as a therapeutic modality to control and treat malignancies. Research approaches include investigation of cytokines, T-cells, antigen-presenting cells, oncolytic viruses and vaccines. For the purpose of this article we concentrate on clinical advances in checkpoint inhibition, primarily cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors and programmed cell death 1 (PD-1) inhibitors. We explore toxicities of treatment and present some of the recent clinical trial data pertaining to these agents.
Immune checkpoint inhibitors
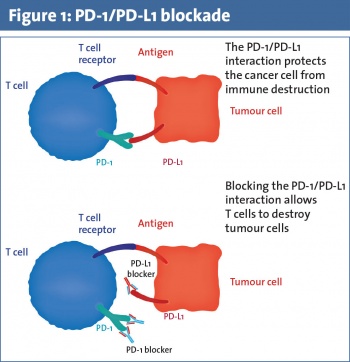
CTLA-4 was discovered in 1987. It is stimulated by antigen presenting cells (APCs) and acts to prevent CD4+ and CD8+ T-cell activation.4 Ipilimumab was the first anti-CTLA-4 antibody immune checkpoint inhibitor to be approved based on its ability to prolong survival in patients with metastatic melanoma.5 PD-1 is a transmembrane protein expressed on T-cells, B-cells, and natural killer (NK) cells. Binding of PD-1 to its ligand PD-L1 inhibits apoptosis of the tumour cell and promotes peripheral T effector cell exhaustion (see Figure 1).6,7 Nivolumab and pembrolizumab are PD-1 antibodies that inhibit tumour cell evasion of the immune system. They are approved for use in patients with advanced melanoma and non-small cell lung cancer as they have demonstrated an overall survival benefit.
Currently several clinical trials are investigating immunotherapy combinations. Concurrent CTLA-4 and PD-1 blockade with ipilimumab plus nivolumab has demonstrated increased overall response rates but this has been associated with increased toxicity; 55% of patients on a pivotal phase 3 trial in melanoma experienced grade 3-4 adverse events such as diarrhoea, deranged liver function tests, and colitis.8
Toxicities
Immune-related adverse events (irAEs) occur due to generalised stimulation and enhancement of the immune system.9,10 The most frequently seen irAEs involve skin (rash), GI tract (diarrhoea/colitis), liver (elevations AST and ALT) and endocrine (hypophysitis, hypothyroidism, hyperthyroidism, thyroiditis or adrenal insufficiency).9 Pneumonitis can also occur. The incidence of pneumonitis appears to be less than 10% in patients receiving anti-PD-1/PD-L1 therapy and is more common in patients with lung cancer.11, 12, 13,14
Rapid identification and early intervention is critical to the successful management of toxicities. Combination immunotherapy is associated with a significantly increased risk of irAEs. For patients experiencing moderate (grade 2) irAEs, management requires withholding the offending immunotherapy agent. Systemic corticosteroids should be commenced if symptoms do not resolve within a week (prednisone 0.5mg/kg/day).15
For life-threatening irAEs (≥ grade 3), management involves permanent discontinuation of the immunotherapy agent and early addition of high doses of corticosteroids (prednisone 1 to 2mg/kg/day or equivalent), followed by a slow steroid taper over at least one month.16
Mortality in immune-related colitis had been associated with delayed reporting, noncompliance with an antidiarrhoeal regimen, and lack of drug withholding.15 Infectious causes must first be ruled out in someone presenting with severe diarrhoea. Once ruled out, corticosteroids should be commenced early, and if not controlling the colitis, consideration should be given to infliximab (5mg/kg) treatment to suppress the unregulated immune system.17,18,19
Immunotherapeutic advances: ASCO 2016
The annual ASCO meeting, with over 5,000 research submissions and more than 36,000 attendees, concentrates the most recent advances in global cancer research. Immunotherapy continues to be investigated and developed across many cancer subtypes. In fact, ASCO identified immunotherapy as the advancement of the year in its Clinical Cancer Advances 2016 report.
“Every year, thousands of oncologist and millions of patients all over the world await the news coming from this meeting -– new breakthroughs, new therapies, new promises of cure, hope. ASCO is harnessing the collective wisdom of oncologists around the world to put patients at the centre of research and care.”
US vice president Joe Biden, ASCO 2016
Colorectal cancer
At the annual meeting in Chicago this year, a phase 2 study looking at PD-1 blockade in mismatch repair (MMR)-deficient cancers reported on 55 patients with metastatic or locally-advanced MMR-deficient colorectal cancer.20 They had received at least two prior lines of systemic therapy prior to proceeding to pembrolizumab 10mg/kg given two weekly.
The overall response rate (ORR) in MMR-deficient colorectal cancer was 57% versus 0% in MMR-proficient colorectal cancer. The investigators also found that mutational load was a good predictor of response to PD-1 blockade.20 This study is potentially practice-changing as it shows that MMR-deficient colorectal cancers have high and durable responses to anti-PD-1 therapy.
Non-small cell lung cancer
Unprecedented response rates were seen in first-line advanced non-small cell lung cancer (NSCLC) with the combination nivolumab (3mg/kg given two weekly) and low-dose ipilimumab (1mg/kg given 6-12 weekly) achieving an ORR of 39-47%.21
Head and neck cancer
Particularly exciting work has revealed new standards for recurrent and metastatic head and neck cancer. The Checkmate 141 phase 3 clinical trial enrolled over 300 patients with recurrent refractory metastatic head and neck squamous cell carcinoma (HNSCC) who had received previous platinum therapy. They were randomised 2:1 to nivolumab versus investigator’s choice of second-line chemotherapy. The one-year OS in the nivolumab arm was 36% versus 16% in the chemotherapy arm (p = 0.01).22 This represents a new treatment paradigm for this patient cohort.
The Keynote 055 Study was a single-arm phase 2 trial of recurrent HNSCC refractory to platinum and cetuximab. Patients received pembrolizumab 200mg three weekly. The ORR was 18%.23 This represents a new standard of care in platinum refractory patients and approval by the FDA and EMA is pending.
Bladder cancer
Advanced urothelial cancer has been challenging to treat, however two studies of atezolizumab (an anti-PDL-1 monoclonal antibody) have provided long-awaited positive data. In patients who were unsuitable for first-line cisplatin, atezolizumab first-line treatment produced a 12-month OS of 57%.24
A separate phase 2 single-arm trial evaluated atezolizumab in patients who had received previous platinum therapy. In this setting, atezolizumab elicited a 12-month OS of 35%. It was well tolerated and compared favourably to alternative therapies in this space. This has led to the FDA accelerating approval of atezolizumab in post-platinum bladder cancer patients.25
Metastatic melanoma
Updated results from Checkmate 067 data were also presented at ASCO 2016. This was a double-blind phase 3 trial of over 900 patients with unresectable metastatic melanoma randomised to nivolumab and ipilimumab combination versus nivolumab or ipilimumab alone. Objective response rates (ORR) were 57.6% for the combination, 43.7% for nivolumab monotherapy and 19% for ipilimumab monotherapy. PFS at 12 and 18 months is similar between combination treatment and nivolumab monotherapy. It appears there may not be a substantial advantage in adding ipilimumab to nivolumab in terms of improving the rates of complete responses.
Of note, the combination therapy is associated with increased incidence (55%) of grade 3-4 adverse events, which appear to also have increased likelihood of involving multiple organs.8 Managing immune-related toxicity can be challenging and requires knowledge of the effects of immune stimulation across many organ systems, eg. gastrointestinal, endocrine, hepatic, dermatologic, renal and dermatologic. In addition, there is a significant cost implication for the combination treatment and society as a whole is struggling with this.
A phase 2 survival analysis in patients with advanced melanoma who discontinued treatment with nivolumab plus ipilimumab due to toxicity showed similar one-year OS rates between patients who discontinued versus those who continued therapy.26 Patients who experience immune-related adverse events may not need to continue treatment as, if they are going to benefit, they will get the clinical response whether the drug is restarted or not and there is little value in re-exposing patients to potential further toxicity.
At this time, the 12-month OS for patients with advanced melanoma who receive combination immunotherapy is identical to that of nivolumab monotherapy.27
However, as follow-up continues, this may change. The standard of care now remains both anti-PD-1 monotherapy or combination immunotherapy. The sequencing with targeted BRAF MEK inhibition is as yet unclear in those patients with somatic BRAF mutations; clinical trials are ongoing.
Conclusion
The exciting area of immunotherapeutics continues to advance clinical outcomes for cancer patients. Clinical trials are on going in adoptive therapies – activated T-cells and autologous tumour infiltrating lymphocytes (TILS).
Randomised controlled trials are investigating the use of these agents in multiple cancer types and, as these agents become safer to deliver, it looks promising that we will be able to harness the unique power of an individual’s own immune system to continue to improve cancer care for patients.
 (click to enlarge)
(click to enlarge)
