RESPIRATORY
WOMEN’S HEALTH
Managing asthma in pregnancy
Discussing the changing views on asthma in pregnancy over the past 60 years, the risks and management strategies are discussed
June 1, 2013
-
Over the past 50-60 years views on asthma in pregnancy have been changed dramatically – from consideration of it as a very benign condition with no effect on pregnancy and its outcomes1 to acceptance that it represents a significant public health issue.2
Asthma prevalence in pregnant women
Asthma is the most common chronic disease in pregnant women, complicating up to 12.4% of pregnancies almost 10 years ago (from 3.2% in early 1990s), and its rates continue to rise.3,4
This most likely reflects the steady increase in asthma prevalence in the general population over the past few decades, from 9.5 million adults being affected by this disease in 1995 to 17.5 million in 2009 (based on US data).5
If studies included not only patients with physician-confirmed diagnosis of asthma but also those with symptoms over the previous year, prevalence rates would be even higher. Ireland has the fourth-highest prevalence of asthma in the world; 470,000 had a diagnosis of this condition in 2006.6
Maternal asthma effects on pregnancy outcomes
Studies assessing the effects of asthma on pregnancy are mostly cohort studies, more commonly retrospective. Advantages and disadvantages for both are summarised in Table 1. Less frequently, cross-sectional surveys, case series and case-control studies are conducted.
Although the data to date have been conflicting to some extent, overall the literature suggests that asthma in pregnant females:
- Affects both the foetus and mother
- Complicates delivery as well as the pregnancy course
- Is associated with adverse pregnancy outcomes.
The most recent and largest retrospective cohort study7 is based on US data from 12 centres providing electronic medical records and International Classification of Diseases, ninth revision (ICD-9) discharge codes from the intrapartum admissions for 228,562 pregnancies among 208,695 women from 2002 through 2008 (the majority of the cohort [87%] delivered from 2005 through 2007).
This study demonstrated a number of very important findings. It confirmed the known fact that females with asthma are at higher risk of having low birth weight babies (by 16%) and pre-term delivery (by 17%), and Caesarean section (by 16%).
Their risk of developing superimposed pre-eclampsia and eclampsia was found to be higher than for non-asthma patients by 34% and 41%, respectively. The odds for other severe complications were also significantly higher in the presence of asthma: for placental abruption it was 1.22 (95% CI, 1.09-1.36), for haemorrhage 1.09 (95% CI, 1.03-1.16), for pulmonary embolism 1.71 (95% CI, 1.05-2.79) and for maternal ICU admission 1.34 (95% CI, 1.04-1.72).
Interestingly, women with asthma in this study were more likely to be obese (9% versus 6.9%) or severely obese (9.4% versus 5.1%). They were more likely to have other chronic condition (8.2% versus 6.2%); almost twice as likely to have pre-existing diabetes (2.2% versus 1.4%); and twice more likely to smoke (12.2% versus 6.2%).
However, even after adjustment for these and other risk factors, asthma was independently associated with higher odds for nearly all complications of pregnancy and delivery.
There was no difference in postpartum fever and maternal death observed. Increased risk for Caesarean delivery and maternal haemorrhage in asthma patients were also reported in earlier review by Dombrowski.8
Another large recent population-based cohort study from Canada9 based on data from 40,788 pregnancies (13,007 asthmatics) between 1990 and 2002 also showed that the odds for low birth weight (OR 1.41: 95% CI 1.22-1.63) and pre-term delivery (OR 1.64: 95% CI 1.46-1.83) were significantly higher among asthmatic women, as well as for small-for-gestational-age (OR 1.27, 95% CI 1.14-1.41).
The proportions of women with mild, moderate and severe asthma in this study were 82.5%, 12.5% and 5.0%, respectively. The risk of small-for-gestational-age was associated with severe (OR 1.48, 95%CI: 1.15-1.91) and moderate-severity asthma (OR 1.30, 95%CI:1.10-1.55) but not mild.
Interestingly, there were no significant associations found between asthma severity, and pre-term birth and low-birth-weight, indicating that asthma regardless of its severity poses significant risk for adverse pregnancy outcomes.
The relationship between asthma severity and pregnancy outcomes was addressed in more detail in a recent systematic review and meta-analysis by Namazy et al.10
This meta-analysis was based on nine cohort studies published between 1975 and March 2012. In that analysis maternal asthma exacerbations and oral corticosteroid use were associated with low birth weight (RR 3.02, 95% CI 1.87-4.89 and RR 1.41, 95% CI 1.04-1.93, respectively) and pre-term delivery (RR 1.54, 95% CI 0.89-2.69 and RR 1.51, 95% CI 1.15-1.98, respectively).
Moderate-to-severe asthma during pregnancy was shown to increase the risk of small-for-gestational-age (RR 1.24, 95% CI 1.15-1.35) and low birth weight (RR 1.15, 95% CI 1.05-1.26) infants.
This highlights the importance of achieving adequate asthma control during pregnancy. Interestingly, an earlier study by the same group of researchers demonstrated that not using inhaled corticosteroids during pregnancy increased the relative risk for low-birth weight by up to 55%.2
Intra-uterine growth restriction associated with asthma severity was also demonstrated in a prospective study from the US (more than 2,200 pregnant women enrolled|).11 This association may reflect an effect of hypoxia on the foetus.
During this same systematic review of publications dated between 1975 and March 2012, 21 cohort studies12 were identified that assessed other perinatal outcomes, including congenital malformations, neonatal complications and perinatal mortality.
They found that maternal asthma was associated with a significantly increased risk of congenital malformations (relative risk [RR] 1.11, 95% CI 1.02-1.21, I2 = 59.5%), cleft lip with or without cleft palate (RR 1.30, 95% CI 1.01-1.68, I2 = 65.6%), neonatal death (RR 1.49, 95% CI 1.11-2.00, I2 = 0%), and neonatal hospitalisation (RR 1.50, 95% CI 1.03-2.20, I2 = 64.5%).
Importantly, use of bronchodilators and inhaled corticosteroids were not associated with congenital malformation risk.
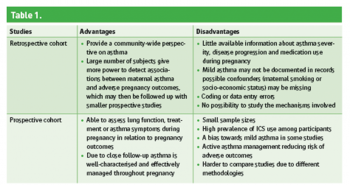 Table 1(click to enlarge)
Table 1(click to enlarge)
