CANCER
NUTRITION
Nutrition in cancer care
The role of parenteral nutrition in the adult cancer patient
November 6, 2019
-
Nutrition is an essential element of multi-modal cancer care1 with the prevalence of cachexia ranging from 60-80% in advanced cancer.2 Recommendations state that individuals should be nutritionally screened on diagnosis and regularly throughout their cancer journey to assess for both risk of, and overt malnutrition.3 This can be affected by cancer type, staging and therapy2 and following dietetic assessment individualised interventions can range from nutritional counselling and oral nutritional supplements to artificial nutrition support in the form of enteral or parenteral nutrition.4
A recent Irish survey of 1085 individuals with cancer found that 44% reported weight loss since diagnosis, with muscle loss noted by 52%, however fewer than half of those surveyed had accessed a dietitian.5 This highlights the need for regular nutritional screening and appropriate referrals as under nutrition is a marker of prognosis while undertaking anti-cancer treatments.5,6 The causes for this impaired intake and associated weight loss are complex and multifactorial;4 the disease itself causes cancer cachexia, which is associated with catabolic alterations such as sarcopenia,1,4 and this is further compounded by side effects of treatment including nausea, vomiting, oral ulceration and chemosensory alteration.4,7
Indications for parenteral nutrition
Parenteral nutrition (PN) refers to the provision of nutrients by the intravenous route.8 “If the gut works, use it” is the principle that guides our approach to nutrition support, however, in cases of total inability to tolerate oral or enteral nutrition (EN) due to factors such as bowel failure, complete obstruction and malabsorption, timely implementation of artificial nutrition support via PN is indicated.4
Previously the term total parenteral nutrition (TPN) was more commonly used and indicated that all macronutrients to include nitrogen, carbohydrate and lipids as well as micronutrients such as trace elements, vitamins and minerals were being met by intravenous nutrition.8 The term PN is now to encompass both total and supplemental nutrition provision via the intravenous route as individuals often remain on PN while an enteral route is being established.9 Although it lacks the benefits of food in the gut,10 one of the advantages of PN over EN is essentially the immediate delivery and easy administration of nutrition once access has been established.11
Prior to considering PN, one must consider the complications associated with this form of nutritional support.12 In comparison to EN, it has potentially higher risks and is more resource intensive, often requiring a higher level of expertise.13 These complications can include hyper- and/or hypoglycaemia, hypertriglyceridaemia and hepatobiliary disorders.14 However, over recent years its advantages have come to the forefront, as decreases in both infectious and non-infectious complications have been demonstrated.15,16
In terms of postoperative care, PN has displayed benefits for both long- and shorter-term use, particularly in cases of impaired gastrointestinal function in patients who were unable to receive and absorb adequate amounts of oral or enteral feeding for at least seven days post-op.17
Oncology and haematology
Medical treatment of cancer generally focuses on the administration of chemotherapy agents and/or radiation therapy.18 For individuals on PN the aim is to enhance tolerance of these and other anti-cancer treatments as well as treating undernutrition in order to improve function and outcome.6
For non-surgical oncology patients, PN would often be initiated in cases of severe mucositis or radiation enteritis as well as supplemental PN being considered in patients when inadequate food and enteral intake (< 60% of estimated energy expenditure) is anticipated for more than 10 days.6
In the haematological malignancy patient group, gastrointestinal complications can occur both during the pre-conditioning treatment phase and post allogenic bone marrow transplant (BMT).19 Both acute and chronic graft versus host disease (GVHD) are known complications of allogenic BMT and when it involves the gut, individuals can suffer with severe abdominal pain and diarrhoea with frequent need for artificial support in the form of PN.19,20
Cancer surgery
In terms of surgical oncology, patients with gastric cancer or cancer-associated obstruction are often susceptible to insufficient oral intake and nutritional depletion.21 According to the European Society of Parenteral and Enteral Nutrition (ESPEN) guidelines, when surgery can be delayed, pre-operative nutritional support for 10-14 days prior to major surgery is recommended for malnourished patients.22
PN is also used routinely in some centres following major gastrointestinal (GI) tract surgery as it has been shown to not only improve the nutritional status of patients with gastric cancer post operatively, but also offers benefits over enteral nutrition in terms of quality of life (QoL) and psychological status.23 Malignant bowel obstructions can occur from direct invasion of the GI tract by the tumour, external pressure of the tumour or adhesions following surgical intervention.7
When enteral nutrition is contraindicated, PN is the first-line nutritional management and may also be used in other post-operative cases to include intestinal ischaemia, malabsorption and high output fistulas.22
Parenteral nutrition preparations
In order to provide nutrients safely and effectively the clinician must understand the components of the PN solution and techniques of administration.24 In those patients with significant weight loss, poor intake and cachexia, calories and protein must be introduced with caution to minimise the risk of refeeding syndrome.17 Three-in-one mixtures including carbohydrate, protein and fat are popular as they allow continuous and stable administration of all necessary components and are convenient as they are not made up on a patient specific basis.17
Less commonly used in Ireland, three-chamber bags provide macronutrients and electrolytes in three separate compartments with the substrates being mixed together immediately prior to intravenous application, this is done by breaking the separation seals between the bag chambers.25
Macronutrient breakdown
In terms of nitrogen provision, all commercially available PN preparations contain the nine essential amino acids.26 ESPEN guidelines state that in illness/stressed conditions individuals may require protein intake up to 1.5g/kg of ideal body weight for adequate nitrogen delivery which is approximately 20% of total energy requirements.17 Considerable emphasis on maintenance of muscle stores is one of the main goals of nutritional therapy as significantly reduced muscle mass is strongly associated with increased complications in cancer surgery and is associated with dose-reducing toxicity during systemic therapy and mortality in cancer patients.4
Many centres are now using ‘new generation’ PN bags containing ‘SMOF’ lipids (soybean oil, medium-chain triglycerides, olive oil and fish oil). Studies have shown that SMOF mixed emulsion is safe and well tolerated with average doses of 1-2g/kg displaying improvements in liver function, antioxidant defences and cholestasis versus using soya bean oil alone.27 Administration of fat intravenously must be closely monitored given the risks of liver toxicity and it is therefore recommended that cholesterol and triglycerides are reviewed weekly initially, to monitor risk of potential hyperlipidaemia.28 Liver damage can progress to liver cirrhosis and failure29 therefore ‘fat free’ regimens are commonly available for those at risk of hypertriglyceridaemia. For patients requiring fat-free PN clinicians should be aware of the possibility of essential fatty acid (EFA) deficiency which can manifest as a skin rash.30 In terms of reference values ESPEN recommend a minimum intake of 7-9g of essential fatty acids daily.31,32
Another approach to reduce fat provision in PN is to increase the glucose:fat ratio of the non-protein calories to reduce the risk of fatty liver, hyperlipidaemia and cholestasis.17 However, increasing the glucose load may not be the best option in this patient group as insulin resistance due to impaired glucose tolerance has been an early finding in cancer patients.33 The stress of trauma, critical illness or major surgery can typically result in insulin resistance and this, along with a large dextrose load from PN, can exceed the glucose oxidation capacity leading to significant hyperglycaemia.34
Studies have shown that PN-induced hyperglycaemia is associated with poor clinical outcome and those individuals with high blood glucose levels during PN often have longer hospital stays. Of interest, it was observed that values before and within 24 hours of initiating PN are better predictors of hospital mortality and complications than blood glucose during the entire duration of TPN.35
In addition, steroids which are known to have a detrimental effect on glycaemic control are often prescribed for both symptom management or as part of a chemotherapy regimen, therefore individuals exposed to medications such as dexamethasone and prednisolone should have their blood glucose monitored regularly.36
Monitoring
Assessment of fluid status is an essential component of determining a PN regimen as electrolyte and water imbalances can have a more profound immediate effect on health than nutrients which can result in both fluid overload and dehydration.32 Monitoring of biochemistry is also a fundamental element of PN prescription: kidney function, bone profile, glycaemic control and lipid profile should be routinely reviewed and C-reactive protein, liver enzymes, bilirubin and albumin also require close surveillance, with micronutrients to include fat soluble vitamins and trace elements being monitored less regularly.32,37
Furthermore, anthropometry and BMI should be checked on each review and weight should be corrected for excessive fluid loads to include pleural effusion, ascites and oedema.4,32,37
Completing this global assessment involves multidisciplinary teamwork, and in order to achieve this collaborative approach to prescribing PN, many centres are now implementing multidisciplinary nutrition support teams.12
Route of access
Multiple mechanisms increase the risk of infectious complications in patients receiving PN, including compounding, central venous catheter care and the PN prescription itself, which can all contribute to catheter-related blood stream infections.38 Administration of PN via central venous access is generally recommended via peripherally inserted central catheters (PICCs) or tunnelled catheters which allow delivery of nutrients directly into the superior vena cava or the right atrium.
Non-tunnelled central venous catheters (CVCs) are often used on a short-term basis during inpatient stays, however the use of the femoral vein is contraindicated due to higher risk of venous thrombosis and potential contamination at the groin.39 Peripheral PN is also used in some centres and may be considered during acute illness as this approach allows early infusion of nutritional substrates without the need to insert a central venous catheter.40 It should only be recommended on a short-term basis and osmolarity of peripheral parenteral nutrition should not be higher than 850mOsmol/l.39
Home parenteral nutrition
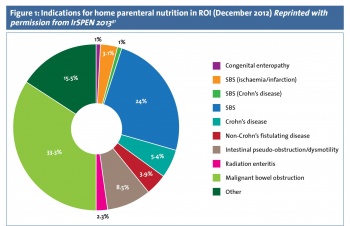
Home PN (HPN) may be indicated when an individual undertaking anti-cancer treatment is suffering from severe complications from chemo/radiotherapy or surgery.32 It has been used with success in Irish National Cancer Centres and in 2012 this patient group accounted for 36% of all home PN in the Republic of Ireland (see Figure 1).41 Survivorship is becoming a key focus for healthcare planning as with advancing treatment options individuals are now both living with and beyond cancer for many years with potential debilitating treatment side-effects.42