CARDIOLOGY AND VASCULAR
Out-of-hospital cardiac arrest
A recent report from the Resuscitation Outcomes Consortium on the wide variability in drug use in out-of-hospital cardiac arrest
May 1, 2013
-
Out-of-hospital cardiac arrest (OHCA) is a serious public health problem with a reported average incidence of 52 emergency medical services (EMS) treated events per 100,000 of the population per year in North America.
Despite the publication and widespread application of international advanced cardiac life support (ALS) guidelines, survival rates remain extremely low. As the lead author of the recent report by the Resuscitation Outcomes Consortium (ROC) published in the journal Resuscitation,1 I wanted to show that there is a huge variability in the use of pharmacological therapies by the emergency medical services in the management of OHCA.
ROC is a North American consortium of research groups engaged in studies in cardiac arrest and severe trauma funded by the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, the Canadian Institutes of Health Research (CIHR), the Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada and the American Heart Association.
The consortium includes 264 EMS agencies across 11 geographically distinct sites. Data related to out-of-hospital treatments and outcomes were collected by use of standardised operational definitions, including initial cardiac rhythms, response times, descriptions of responders, timing of cardiopulmonary resuscitation (CPR) and defibrillation, response to interventions, return of spontaneous circulation (ROSC), the presence of a pulse at arrival to the emergency room and survival to hospital discharge.
Advanced cardiac life support guidelines
The aim of the ALS guidelines is to help to standardise the provision of basic and advanced level care based on the available evidence and expert opinion. In order to help optimise cardiac and cerebral perfusion during CPR, increase defibrillation success and achieve a good neurological outcome, the ALS guidelines recommend the administration of specific anti-arrhythmic and vasoactive drugs under certain conditions. However, interestingly, there are limited data regarding the beneficial effects of many of these agents, which prompted us to examine current practice in more detail.
My colleagues and I studied a total of 16,221 OHCAs which were attended by 74 EMS agencies. This is the largest study describing the contemporary use of pharmacological agents in OHCA.
Anti-arrhythmic drugs
In terms of anti-arrhythmic drugs a large difference in the use of lignocaine and amiodarone was noted with fewer than half of agencies only administering one anti-arrhythmic drug (see Figure 1).1
Within those agencies using lignocaine, the proportion of patients receiving the drug in cases with at least one shock ranged from 1-75%. For those who had shock-resistant arrhythmias, lignocaine administration rates ranged from 1-100% between agencies (mean 41 ± 31%). In agencies using amiodarone, the proportion receiving amiodarone ranged from 0.2-63% for patients who received at least one shock, and from 0.4-90% for shock-resistant ventricular arrhythmias (mean 26 ± 32%).
After adjusting for age, gender, EMS-witnessed arrest, bystander-witnessed arrest, bystander CPR, shockable initial rhythm, time from 911 to EMS arrival and study site, the odds ratio for survival to hospital discharge was 1.11 for amiodarone and 1.28 for lidocaine.
Like all data analysis we must treat this with caution and not draw too many conclusions or extrapolate these data.
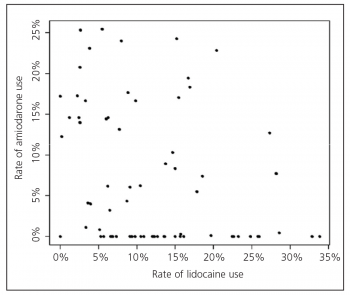 Figure 1. Relation between use of amiodarone and lidocaine for all cardiac arrests across all agencies throughout the Epistry ROC database. Each point represents one agency with the exception of two agencies with zero use of both drugs(click to enlarge)
Figure 1. Relation between use of amiodarone and lidocaine for all cardiac arrests across all agencies throughout the Epistry ROC database. Each point represents one agency with the exception of two agencies with zero use of both drugs(click to enlarge)

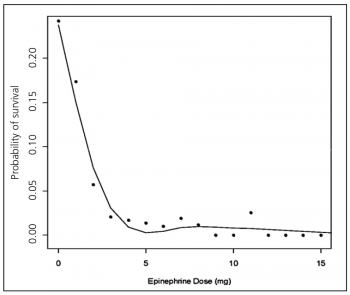 Figure 2. Relation of epinephrine dose to survival. Each point represents the total dose of epinephrine in milligram versus the rate of survival for individuals who received that dose(click to enlarge)
Figure 2. Relation of epinephrine dose to survival. Each point represents the total dose of epinephrine in milligram versus the rate of survival for individuals who received that dose(click to enlarge)