RESPIRATORY
SURGERY
Interstitial lung disease
Interstitial lung diseases encompass a wide range of diffuse pulmonary disorders and multidisciplinary discussion has become the gold standard for diagnosis
February 1, 2013
-
Interstitial lung diseases (ILDs) are characterised by a variable degree of inflammatory and fibrotic changes of the alveolar wall and eventually the distal bronchiolar airspaces. ILDs may occur in isolation or in association with systemic diseases.
The clinical evaluation of a patient with ILD includes a thorough medical history and detailed physical examination; obligatory diagnostic testing includes laboratory testing, chest radiography and high-resolution computed tomography (HRCT), and comprehensive pulmonary function testing and blood-gas analysis. To optimise the diagnostic yield, a dynamic interaction between the clinician, radiologist and pathologist is mandatory.
ILDs are a heterogeneous group of more than 150 disease entities that differ significantly with respect to prevention, therapy and prognosis. The diagnostic strategy in a patient with ILD is based on considerations regarding the dynamic time course (acute, subacute, chronic), the cause (known or unknown) and the context of the disease at presentation (presence of extrapulmonary/systemic disease manifestations).
Once an interstitial disease process has been recognised in a patient, there are three crucial questions that have to be addressed in the diagnostic work-up:
- Is there a discernible cause for the disease?
- If no cause is identifiable, is it idiopathic pulmonary fibrosis (IPF)?
- If there is no cause of the disease and if it is not IPF, should surgical lung biopsy be recommended?
After a diagnosis has been established, the severity and dynamics of the disease have to be assessed and monitored, with or without therapy. Diagnosis and disease severity/dynamics are fundamental for treatment decisions and to predict prognosis. The diagnostic approach to ILD may have to be adapted to different clinical scenarios that eventually lead to presentation of a patient:
- Patient presents with clinical symptoms (eg. dry cough, dyspnoea)
- Patient is at risk of ILD due to known exposures (eg. amiodarone, asbestos)
- Patient is at risk of ILD due to family history
- Patient is asymptomatic, but presents with chance finding on chest radiography or computed tomography
- Patient is asymptomatic, but presents with chance finding on pulmonary functioning test.
History taking
A comprehensive patient history taking is of crucial importance for the diagnosis of ILD. There are four main questions to be answered. When did respiratory symptoms start? How did the disease develop over time to the present? Are there or have there been any exposures to aetiologic agents known to cause ILD? What is the severity of symptoms at presentation?
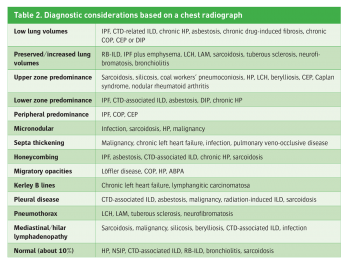
The disease chronology can be subdivided into four categories: acute – days up to a few weeks; subacute – four to 12 weeks; chronic – longer than 12 weeks; and episodic – symptomatic phases that are followed by asymptomatic phases. In addition, all available radiographs of the lung should be reviewed to characterise the nature and development of the radiological pattern. Flitting opacities on chest imaging studies may drive the differential diagnosis to focus on eosinophilic pneumonia, hypersensitivity pneumonitis (HP), vasculitis or organising pneumonia.
Assessment of symptoms
Dyspnoea on exertion or at rest is the predominant symptom in most ILDs. Cough is the second most frequent symptom in patients with ILD and sometimes becomes really bothersome. Although a dry cough is common in IPF, cough is generally an airway symptom and therefore more indicative of airway-centred diseases such as sarcoidosis, HP or organising pneumonia.
Pleural pain and effusion in the context of an ILD indicate connective tissue disease (eg. systemic lupus erythematosus [SLE] or rheumatoid arthritis [RA]) or drug-induced or asbestos-related disease. Differential diagnoses include complications such as infections or pulmonary embolism.
Haemoptysis is always an alarming signal and may indicate manifestation of pulmonary haemorrhagic syndromes, for example, Goodpasture syndrome or granulomatosis with polyangiitis (GPA, previously called Wegener disease).
Alternatively, infections, lung cancer or pulmonary embolism have to be considered. Gastro-oesophageal reflux is another common symptom in patients with ILD that is suspected of causing or at least exacerbating ILD. A history of acid reflux should, therefore, be taken in all patients with ILD. Extrapulmonary features of associated diseases may provide important hints to the correct diagnosis.
Therefore, the following should be carefully sought:
- Joint pain and swelling (RA) and cutaneous thickening
- Raynaud’s phenomenon and dysphagia (systemic sclerosis)
- Oculocutaneous albinism and colitis (Hermansky-Pudlak syndrome)
- Chronic granulomatous sinusitis (GPA and Churg-Strauss syndrome)
- Renal failure (Goodpasture syndrome)
- Renal angiomyolipoma (lymphangioleiomyomatosis)
- Crohn’s disease.
Physical examination
On physical examination, inspection of the integument may reveal valuable findings:
- Skin thickening and acral necrosis (scleroderma)
- Oculocutaneous albinism (Hermansky-Pudlak syndrome)
- Clubbing (up to 40% in all ILDs, up to 66% in IPF)
- Livedo racemosa (SLE)
- Cutaneous vasculitis (Churg-Strauss syndrome)
- Oedematous-cyanotic skin (dermatomyositis, ‘disease lilac’).
Palpation may reveal lymphadenopathy, hepatosplenomegaly pointing at sarcoidosis, HIV infection or connective tissue disease. On auscultation of the lungs, symmetric fine ‘Velcro-like’ inspiratory crackles are found in more than 90% of patients with IPF and in about 60% of patients with connective tissue disease-associated ILD.
Crackles are less frequent in HP and rare in sarcoidosis. Wheezing and inspiratory squeaks reflect bronchiolitis and/or bronchial obstruction and are associated with Churg-Strauss syndrome, HP and, rarely, non-specific interstitial pneumonia.
Laboratory testing
There are no specific laboratory tests that allow for the diagnosis of an ILD. Routine laboratory testing should include:
- A complete blood cell count
- Leukocyte differential
- Platelet count
- Erythrocyte sedimentation rate
- Determination of serum electrolyte levels, including calcium, serum urea nitrogen and creatinine
- Liver function tests
- Urinary sediment.
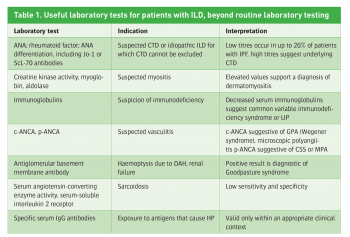
These laboratory values allow the exclusion or suggestion of an associated haematological, liver or kidney disease in a potential context of systemic disease (eg. sarcoidosis, vasculitis, amyloidosis), malignancy (eg. lymphoma) or infection (eg. tuberculosis, HIV). To further evaluate the presence of connective tissue disease, systemic disease (eg. sarcoidosis, connective tissue disease) or HP, additional measures may be appropriate as summarised in Table 1.
 (click to enlarge)
(click to enlarge)

 (click to enlarge)
(click to enlarge)