ENDOCRINOLOGY
GASTROENTEROLOGY
NEUROLOGY
Delayed hepatocellular damage with rhabdomyolysis
A Dublin hospital reported on a case of delayed hepatocellular damage with rhabdomyolysis, following exertional heat stroke that developed during a half marathon
February 1, 2015
-
We report a 41-year-old man who developed rhabdomyolysis and severe hepatocellular damage following a run of 9km. He made a full clinical recovery with conservative management. The case highlights the clinical features of his presentation. It demonstrates that exertional heat stroke (EHS) can occur even in temperate climates, that clinical recovery is possible with conservative management, and it highlights the significance of early body cooling and serial monitoring of biochemical parameters.
EHS is a medical emergency, requiring prompt recognition and treatment, as it is associated with life threatening complications.1 Early recognition with immediate cooling can ameliorate the elevated core body temperature; and this may prevent organ failure and improve prognosis.1
Case report
A previously healthy 41-year-old man collapsed at the 9km mark while partaking in a half marathon. Past history was significant for a similar episode of collapse but with no sequelae. On this occasion, he lost consciousness, but regained consciousness during transfer to the emergency department (ED) via a standby ambulance.
Examination revealed a clinically dehydrated, disorientated individual without focal neurological deficits. His tympanic temperature measured 38.5oC. He was tachypnoeic at 34 breaths per minute and had a sinus tachycardia on electrocardiogram of 133 beats/minute. His Glasgow coma scale (GCS) was 13/15. Bed side capillary blood glucose was 9.8mmol/l and troponin I <0.05ng/ml.
A preliminary diagnosis of EHS was made. He was treated immediately with fluid resuscitation and body cooling with ice packs. Blood profiles were normal for blood counts, liver tests, urea, electrolytes and coagulation. Mild elevation of serum creatinine 154µmol/L. Arterial blood showed lactate 6.8mmol/L, pH 7.4 and bicarbonate 24.3mmol/L. He was discharged once his vital signs and GCS normalised.
He returned three days later with fatigue and vomiting. GCS, vital signs and tympanic temperature were normal. Scleral icterus was seen without organomegaly or hepatic encephalopathy.
Investigations revealed severe hepatocellular damage with elevated creatine kinase (CK) (see Figure 1). Total bilirubin 115µmol/L, gamma-glutamyl transpeptidase 139 IU/L, lactate dehydrogenase 2945 IU/L (< 500 IU/L), and platelet count 115 x 109/L (150 to 400), international normalised ratio (INR) 1.69 and prothrombin time (PT) 19.4 seconds, lactate 0.9mmol/L, pH 7.4, bicarbonate 24.3mmol/L and normal renal function.
Investigations for hepatitis were negative and included: urine and serum toxicology, serology for viral hepatitis, leptospirosis, alpha1-antitrypsin, ceruloplasmin, immunoglobulin levels and autoantibody screening. Ultrasonography revealed a grossly normal liver with no large vessel thrombosis. Holter monitor showed sinus rhythm with heart rate varying from 33 to 129 beats/min and no malignant arrhythmia. Gradual improvements in laboratory parameters were seen. Clinical recovery was observed by the 12th day. CK had decreased to 167 IU/L on the 26th day, and liver blood tests were normal by day 30.
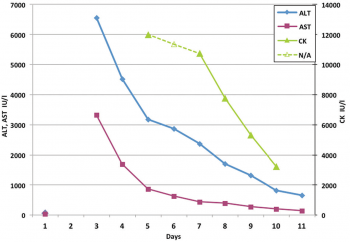 Figure 1: Line graph demonstrating differential improvement in hepatic enzymes and CK, creatine kinase. AST, aspartate aminotransferase (normal < 42 IU/L), ALT, alanine aminotransferase (normal < 40 IU/L)(click to enlarge)
Figure 1: Line graph demonstrating differential improvement in hepatic enzymes and CK, creatine kinase. AST, aspartate aminotransferase (normal < 42 IU/L), ALT, alanine aminotransferase (normal < 40 IU/L)(click to enlarge)
