SURGERY
UROLOGY
Understanding bladder voiding dysfunction
To understand the concept of bladder dysfunction, it is important to remember the mechanism by which the bladder develops, and its relationship to the nervous system and adjacent structures
March 1, 2015
-
The bladder is an incredibly complex organ, which develops from the anterior cloaca, a structure that has exquisite evolutionary conservatism, as it performs a vital task across phyla of species from birds to reptiles to mammals, with only small adjustments across species. Interestingly, humans are one of the only species to actually suffer from bladder dysfunction, which demonstrates the obvious and complex links with high order centres.
Bladder embryology
During foetal development, the terminal part of the hindgut ends in the cloaca, which is an endoderm-lined chamber that contacts the surface ectoderm at the cloacal membrane and communicates with the allantois, which is a membranous sac that extends into the umbilicus alongside the vitelline duct. The cloaca is then divided by the urorectal septum with the dorsal (inferior) portion developing into the rectum and anal canal, and the ventral (superior) portion developing into the bladder and urogenital sinus, which will give rise to the bladder and lower urogenital tracts (prostatic and penile urethrae in males; urethra and lower vagina in females). As the bladder grows and expands, the distal ends of the mesonephric ducts are absorbed into the wall of the bladder as the trigone. Malformations during development (aside from a condition involving failed reinforcement of the cloacal membrane by mesoderm, such as bladder exstrophy) can lead to a number of conditions such as abnormal ureter attachments, urachal fistulas, sinuses and cysts.
Bladder anatomy and physiology
The bladder acts not only as a temporary store for urine before its coordinated expulsion through the urethra at an appropriate time and place, but also as an autocrine organ involved in its own regulation.
The bladder is the most anterior element of the pelvic viscera. It is situated in the pelvic cavity when empty, but expands superiorly into the abdominal cavity when full. The urinary bladder is abdominal at birth, positioned at the extraperitoneal area of the lower abdominal wall. At around the fifth or sixth year of age, the bladder gradually descends into the area of the true (minor) pelvis. It contains four main parts: the apex, the base, and the superior and inferolateral surfaces. The mucosal lining on the base of the bladder is smooth and firmly attached to the underlying smooth muscle coat of the wall – unlike elsewhere in the bladder where the mucosa is folded and loosely attached to the wall. The smooth triangular area between the openings of the ureters and urethra on the inside of the bladder is known as the trigone.
The vesicoureteric junctions is found as the ureter approaches the bladder. At 2cm to 3cm from the bladder, a fibromuscular sheath (of Waldeyer) extends longitudinally over the ureter and follows it to the trigone. The ureter pierces the bladder wall obliquely, travels 1.5cm to 2cm, and terminates at the ureteral orifice. As it passes through a hiatus in the detrusor (intramural ureter), it is compressed and narrows considerably. The intravesical portion of the ureter lies beneath the urothelium, it is backed by a strong plate of detrusor muscle. With bladder filling, this arrangement is thought to result in passive occlusion of the ureter, like a flap valve.
This anatomic arrangement helps prevent reflux during bladder filling by fixing and applying tension to the ureteral orifice. As the bladder fills, its lateral wall telescopes outward on the ureter, thereby increasing intravesical ureteral length. Vesicoureteral reflux is thought to result from insufficient submucosal ureteral length and poor detrusor backing. Chronic increases in intravesical pressure resulting from bladder outlet obstruction can cause herniation of the bladder mucosa through the weakest point of the hiatus above the ureter and produce a ‘Hutch diverticulum’ and reflux.
One of the reasons that bladders can work so efficiently irrespective of the stimulus, is as a result of transitional cell epithelium, which is waterproof and distensible, and allows bladder filling without a concomitant rise in pressure (compliance). Bladder filling from the excretion of urine by the kidneys occurs via the ureters. The walls of the ureters contain smooth muscle arranged in spiral, longitudinal and circular bundles, but distinct layers of muscle are not seen. Regular peristaltic contractions occurring one to five times per minute move the urine from the renal pelvis to the bladder, where it enters in spurts synchronous with each peristaltic wave. The ureters pass obliquely through the bladder wall and, although there are no ureteral sphincters as such, the oblique passage tends to keep the ureters closed except during peristaltic waves, preventing reflux of urine from the bladder. An adult bladder should hold somewhere between 400ml and 600ml, whereas children require a separate calculation: Expected bladder capacity = [30 + (age in years x 30)] ml.
During bladder emptying, contraction of the circular muscle, which is called the detrusor muscle, is mainly responsible. Muscle bundles pass on either side of the urethra and these fibres are sometimes called the internal urethral sphincter (smooth muscle), although they do not encircle the urethra. Farther along the urethra is a sphincter of skeletal muscle, the sphincter of the membranous urethra, external urethral sphincter. This needs to happen in an extremely coordinated manner as described below, and micturition is therefore fundamentally a spinal reflex, facilitated and inhibited by higher brain centres and, like defecation, subject to voluntary facilitation and inhibition (see Table 1).
Bladder filling and a desire to void stimulates afferent impulses to the sacral micturition centre in the spinal cord which allows for sphincter relaxation via the pudendal nerve (lesions lead to incomplete evacuation), as well as afferents to the pontine micturition centre in the pons, which facilitate detrusor contraction and synchronisation. The cortex acts in its role as a higher order centre to determine a socially acceptable situation for micturition and has inhibitory control of the pons.
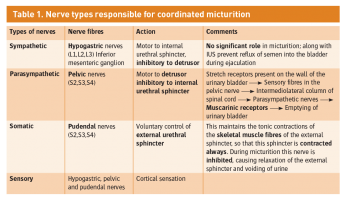 Table 1. Nerve types responsible for coordinated micturition(click to enlarge)
Table 1. Nerve types responsible for coordinated micturition(click to enlarge)
